Lead iodide PbI2
99.999% (trace metals basis),perovskite grade
Lead iodide (chemical formula: PbI2) is the most common iodide of lead. It is a yellow solid at room temperature and turns brick red upon heating. It has low solubility in water but
is pbi2 soluble in water.Lead ii iodide formula: PbI2
The molecular or chemical formula of Lead Iodide is PbI2. Plumbous Iodide is a crystalline solid bright yellow in colour at room temperature.
Lead(II) iodide is a chemical compound with the formula PbI ₂. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide. Wikipedia
- Lead(II) iodidePropertiesLead iodide formula:PbI2Molar mass:461.01 g·mol⁻¹Appearance:Yellow solidDensity:6.16 kg/LMelting point:402°C (675 K)Boiling point:872°C (1145 K)Solubility in water:0.62 g/L at 20°CBandgap:2.34 eV (direct)Magnetic susceptibility:-126.5 × 10^-6 cm³/molHazardFlashpoint:Non-flammable
- Lead(II) iodide Catalog
Lead(II) iodide
-

Lead iodide PbI2
-
Lead iodide PbI2 solubility

Lead iodide PbI2 solubility
-
Lead iodide PbI2 device efficiency (CVD in the figure represents 99.99% lead iodide crystal):
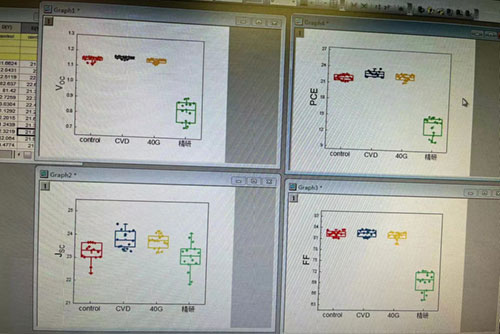
Lead iodide PbI2 device efficiency (CVD in the figure represents 99.99% lead iodide crystal
-
Lead(II) iodide
-

Lead iodide PbI2
-
Lead iodide PbI2 solubility

Lead iodide PbI2 solubility
-
Lead iodide PbI2 device efficiency (CVD in the figure represents 99.99% lead iodide crystal):

Lead iodide PbI2 device efficiency (CVD in the figure represents 99.99% lead iodide crystal
-
-
Why is lead II iodide yellow?
Lead(II) iodide, also known as lead iodide, is a yellow-colored solid at room temperature. The yellow color of lead iodide arises due to its electronic structure and its absorption of visible light.
Lead iodide has a crystal lattice structure, with each lead ion (Pb2+) surrounded by six iodide ions (I-). The yellow color of lead iodide is due to its ability to absorb light in the blue-green region of the visible spectrum. This absorption occurs because the energy of the photons in the blue-green region of the spectrum is close to the energy required to excite electrons in the lead iodide crystal from the valence band to the conduction band.
Lead iodide synthesis
When a photon of light with the appropriate energy is absorbed by the crystal, an electron in the valence band is excited to the conduction band, leaving behind a hole in the valence band. The electron-hole pair, or exciton, interacts with the crystal lattice and eventually recombines, releasing the excess energy as a photon of light. The energy of the emitted photon is slightly lower than that of the absorbed photon, resulting in a yellow color.
The yellow color of lead iodide is due to the absorption of blue-green light by the crystal, which results in the excitation of electrons from the valence band to the conduction band and the subsequent emission of yellow light when the electrons recombine with holes in the valence band. -
Why is lead chloride white but lead iodide yellow?
Lead chloride and lead iodide are both inorganic compounds, but they have different chemical structures and properties. Lead chloride (PbCl2) is is pbi2 soluble, white, water-soluble, compound that forms when lead (II) oxide is treated with hydrochloric acid. It is a common reagent in laboratory experiments and is used in the production of other inorganic compounds.Lead iodide (PbI2), on the other hand, is a yellow, water-insoluble compound that forms when lead (II) oxide is treated with hydroiodic acid. It is a semiconductor material and is used in electronic devices such as solar cells and radiation detectors.The color difference between the two compounds is likely due to the different crystal structures and electronic properties of the two compounds. Lead chloride has a simple crystal structure, while lead iodide has a more complex crystal structure. This results in different absorption and scattering of light, which leads to the different colors.
the electronic properties of lead chloride and lead iodide differ mainly due to their different crystal structures and resulting differences in electronic polarization. Lead chloride, with its symmetrical tetrahedral arrangement of ions, acts as a good insulator, while lead iodide, with its more complex octahedral arrangement of ions, exhibits semiconducting behavior. These differences in electronic properties also influence their optical properties, with lead chloride being relatively transparent and lead iodide appearing yellow.
-
What is the difference in electronic properties between lead chloride and lead iodide?
The electronic properties of lead chloride and lead iodide differ due to the different arrangements of their constituent atoms and the resulting differences in their crystal structures.
Lead chloride has a simple crystal structure in which each lead ion is surrounded by four chloride ions in a tetrahedral arrangement. The lead ions in lead chloride have a +2 oxidation state and are surrounded by a symmetrical arrangement of negative chloride ions. This symmetry results in a lack of electronic polarization and a relatively low dielectric constant. The electronic properties of lead chloride make it a good insulator.
Lead iodide, on the other hand, has a more complex crystal structure in which each lead ion is surrounded by six iodide ions in an octahedral arrangement. The lead ions in lead iodide also have a +2 oxidation state, but the iodide ions are arranged in a less symmetrical manner than the chloride ions in lead chloride. This asymmetry results in a higher degree of electronic polarization and a higher dielectric constant than lead chloride. The electronic properties of lead iodide make it a semiconductor material.
The differences in electronic properties between lead chloride and lead iodide also affect their optical properties and give rise to their different colors. Lead chloride is a relatively transparent compound, while lead iodide is yellow due to its ability to absorb light in the visible spectrum.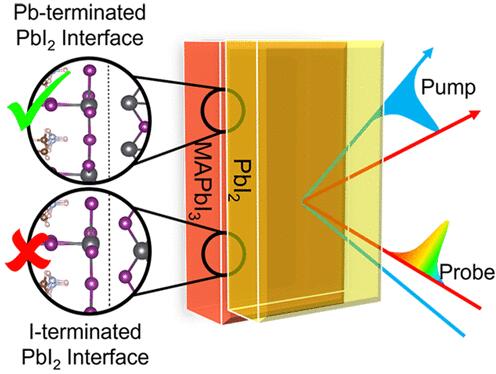
Lead Iodide Perovskite Solar Cells
The electronic properties of lead chloride and lead iodide differ due to the different arrangements of their constituent atoms and the resulting differences in their crystal structures.
Lead chloride has a simple crystal structure in which each lead ion is surrounded by four chloride ions in a tetrahedral arrangement. The lead ions in lead chloride have a +2 oxidation state and are surrounded by a symmetrical arrangement of negative chloride ions. This symmetry results in a lack of electronic polarization and a relatively low dielectric constant. The electronic properties of lead chloride make it a good insulator.
Lead iodide, on the other hand, has a more complex crystal structure in which each lead ion is surrounded by six iodide ions in an octahedral arrangement. The lead ions in lead iodide also have a +2 oxidation state, but the iodide ions are arranged in a less symmetrical manner than the chloride ions in lead chloride. This asymmetry results in a higher degree of electronic polarization and a higher dielectric constant than lead chloride. The electronic properties of lead iodide make it a semiconductor material.
The differences in electronic properties between lead chloride and lead iodide also affect their optical properties and give rise to their different colors. Lead chloride is a relatively transparent compound, while lead iodide is yellow due to its ability to absorb light in the visible spectrum.
-
What makes lead II iodide?
Plumbous iodide is commonly produced by a double displacement reaction between lead nitrate and potassium iodide in water. At room temperature, potassium nitrate is soluble, whereas lead iodide is insoluble, and therefore it undergoes precipitation.
Organic-Inorganic Perovskite Precursors
TCI strongly supports the research and development of perovskite with a wide variety of high quality precursors and scale up capabilities.
High Purity / Very Low Metal Content / Product Variety / Scale-Up
"Perovskite" originates from the mineral name of calcium titanate (CaTiO3) and the compounds with formula of ABO3 generally belong to a perovskite-type compound, where the A is a divalent and B is a tetravalent metal ion. A perovskite with cubic or orthorhombic phases shows ferroelectricity, for instance, barium titanate (BaTiO3) is a ferroelectric or piezoelectric material. High temperature superconductive oxides with unit of a copper oxide are obtained from all perovskite compounds. These perovskite compounds consist of metal ions and oxygen atoms, and are manufactured by a physical procedure (eg. sintering method). Modification of the metal ion and a changing ratio of the metal ion components can drastically control physical properties of the perovskite. In addition to the oxide perovskites, halide-based perovskites are also well known.
On the other hand, one can replace the cationic component with an organic ammonium. In this case, a chemical method can provide a perovskite compound. This perovskite compound is called an ‘organic-inorganic perovskite compound’, because it contains an organic component. A metal ion component usually involves tin or lead.4,5) This perovskite compound has the general formula [(RNH3)mMXn], in which modifications of metal (M), halide (X) and organic groups (R) precisely control physical properties. Among them, the tin perovskite is relatively better for electrical conduction,6) and the lead one is better for optical properties. A chemical modification of the halide controls band gap.8) Selection of organic ammonium halide, metal halide and their mixing ratio changes the component ratio of the halide. The organic groups are selected from methyl, long alkyls, phenyl, benzyl, phenethyl and so on. Diversity of these organic groups allows controlling the structure of a perovskite compound. For instance, a perovskite compound with R = methyl provides [(MeNH3)MX3] having a three-dimensional cubic perovskite structure. A perovskite compound with R = CnH2n+1 (n ≥ 2) provides a two-dimensional perovskite layer and the length of alkyl group can control the inter-layer distance.
References
- 1) E. Sawaguchi, Y. Akishige, M. Kobayashi, J. Phys. Soc. Jpn. 1985, 54, 480.

- 2) Y. Tokura, H. Takagi, S. Uchida, Nature 1989, 337, 345.

- 3) F. S. Galasso, M. Kestigan, Inorg. Synth. 1973, 14, 142.

- 4) D. B. Mitzi, C. A. Feild, W. T. A. Harrison, A. M. Guloy, Nature 1994, 369, 467.

- 5) K. Liang, D. B. Mitzi, M. T. Prikas, Chem. Mater. 1998, 10, 403.

- 6) Y. Takahashi, R. Obara, Z.-Z. Lin, Y. Takahashi, T. Naito, T. Inabe, S. Ishibashi, K. Terakura, Dalton Trans. 2011, 40, 5563.

- 7) N. Pellet, P. Gao, G. Gregori, T.-Y. Yang, M. K. Nazeeruddin, J. Maier, M. Grätzel, Angew. Chem. Int. Ed. 2014, 53, 3151.

- 8) S. A. Kulkarni, T. Baikie, P. P. Boix, N. Yantara, N. Mathews, S. Mhaisalkar, J. Mater. Chem. A 2014, 2, 9221.

- 9) Y. Kawamura, H. Mashiyama, K. Hasebe, J. Phys. Soc. Jpn. 2002, 71, 1694.

- 10) T. Ishihara, J. Takahashi, T. Goto, Phys. Rev. B 1990, 42, 11099.

- 11) A. Kojima, K. Teshima, Y. Shirai, T. Miyasaka, J. Am. Chem. Soc. 2009, 131, 6050.

- 12) J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin, M. Grätzel, Nature 2013, 499, 316.

- 13) M. Liu, M. B. Johnston, H. J. Snaith, Nature 2013, 501, 395.

- 14) H. Zhou, Q. Chen, G. Li, S. Luo, T.-B. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu, Y. Yang, Science 2014, 345, 542.

- 15) W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo, S. I. Seok, Science 2015, 348, 1234.

- 16) G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herza, H. J. Snaith, Energy Environ. Sci. 2014, 7, 982.

- 17) M. Saliba, T. Matsui, J.-Y. Seo, K. Domanski, J.-P. Correa-Baena, M. K. Nazeeruddin, S. M. Zakeeruddin, W. Tress, A. Abate, A. Hagfeldtd, M. Grätzel, Energy Environ. Sci. 2016, 9, 1989.
- 1) E. Sawaguchi, Y. Akishige, M. Kobayashi, J. Phys. Soc. Jpn. 1985, 54, 480.






