Linear Formula: PbI2
CAS Number: 10101-63-0
Molecular Weight: 461.01
EC Number: 233-256-9
Lead(II)iodide is a wide bandgap (2.32 eV) semiconductor material. It has uniqueproperties like high resistivity, chemical stability, and a wide range oftemperature applications (−200 °C up to +130 °C). Perovskites with lead as the central cation produce the bestphotovoltaic efficiency. We offer lead iodide, specifically designed forenhanced solar cell performance.

Lead iodide PbI2

Lead iodide PbI2 solubility
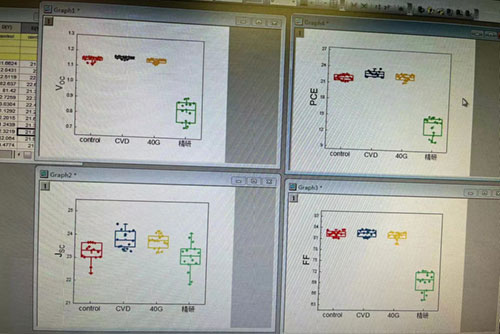
Lead iodide PbI2 device efficiency (CVD in the figure represents 99.99% lead iodide crystal
Application
Lead iodide finds application in synthesis of perovskites based photovoltaic materials. Our perovskite grade PbI2 can readily be dissolved in DMF to yield 1M solution.
Solution-processible Perovskite Solar Cell Device Using Highly Pure Lead Iodide
It is also known as plumbous iodide, lead (II) iodide, or lead diiodide.
At room temperature, lead iodide is a bright yellow, odorless, crystalline solid. When heated, it turns orange and red.
Lead iodide is almost insoluble and precipitates
Lead iodide is the most common iodide of lead. It is an ionic compound.
Lead iodide is harmful if swallowed or inhaled. It may cause cancer, damage fertility or the unborn child, and damage organs through prolonged or repeated exposure. It is also very toxic to aquatic life.
Borun New Material is a manufacturer of perovskites , which are organic optoelectronic materials. Perovskites have unique properties, such as high resistivity and chemical stability.
Is PbI2 an element or compound?
PbI2 is an ionic compound with the formula PbI2. It is also known as lead(II) iodide or plumbous iodide.
PbI2 is a bright yellow crystalline solid at room temperature. When heated, it becomes orange and red. It has a wide band gap value of 2.27 eV.
PbI2 is used in the manufacture of solar cells, X-ray and gamma-ray detectors. It is also used in a patented photographic process. It was formerly used as a yellow pigment in some paints, but this use has been largely discontinued due to its toxicity and poor stability.
PbI2 is made up of two chemical elements: lead (Pb) and iodine (I). Lead carries a +2 charge and forms an ionic bond with iodine to form the compound.
What is Lead lodide?
Plumbous Iodide or commonly known as Lead Iodide is a salt which has a widespread application as a solvent, in the medical industry and the photography industry. Essentially, Lead Iodide is derived from the reaction of potassium nitrate and Lead Iodide through the process of double displacement. Universally, the Lead Iodide formula is PbI2. It’s physical as well as chemical properties make it ideal for a wide array of applications in the printing, medical and industrial solvents realm. It is often interchangeably referred to as lead diiodide due to its structure.
PbI2 colour is yellow crystalline solid.
It is odourless.
Lead Iodide has a density of 6.16 g/cm3.
Lead Iodide is denser than water and is insoluble in most liquids, barring boiling water and potassium iodine.
The melting point of Lead iodide is 402 °C.
The boiling point is 953 °C.
The molecular weight of Lead Iodide is 461.01 g/mol
The thermal conductivity is 26.864.3 10-3 W cm-1 K
Lead Iodide is non-combustible
When looked through X-ray powder diffraction, Lead Iodide resembles a hexagonal close-packed system. It alternates between layers of iodine and lead atoms. It is mostly ionic bonds with weak Van der Waals interactions. Lead iodide solid can also take a rhombohedral structure.
What are the Chemical Properties of Lead Iodide?
Lead Iodide belongs to the Lead-14 and the Iodine-17 group and is a part of the P-3mI symmetrical group. As mentioned earlier the chemical formula of Lead Iodide is PbI2. Lead Iodide is highly resistant to a large spectrum of temperature and portrays extreme chemical stability. This makes it an ideal ingredient for film development and also as an oxidizing agent.
In ambient air (oxygen), thin films of Lead Iodide, however, is quite unstable and forms iodine.
2 PbI2 + O2 → 2 PbO + 2 I2↑
It has a molar mass of 461.01 g/mol.
Lead(II) iodide reacts with concentrated sulfuric acid to produce lead(II) sulfate, hydrogen sulfide, iodine and water.
4PbI2 + 5H2SO4 (conc.) → 4PbSO4 + 4I2 + H2S +4H2O
Now that we know the properties of Lead Iodide, let us now understand the process of the formation of Lead Iodide.
How is Lead Iodide prepared?
The chemical formula Lead Iodide involves the double displacement of soluble lead nitrate and potassium iodine. The product of this chemical reaction is a yellow, colourless solid Lead Iodide and the by-product is a potassium nitrate solution. This follows the chemical principle that when two soluble salts of the metal group react with each other, one soluble salt is formed along with an insoluble metal salt. In our case, the later is PbI2 or Lead Iodide. This method is known as the Bridgman-Stockbarger method.
Pb(NO3)2 + 2 KI → PbI2 + 2 KNO3
This insoluble metal salt precipitates as yellow crystals and is recovered through the process of filtration. This is referred to as the “golden rain”. Typically, this filtration process involves washing the Lead Iodide precipitate in cold water. However, if they are dissolved in hot water and then allowed to recrystallise, they form fascinating golden crystals of Lead Iodide. Care must be taken that any amount of soluble Lead Iodide is removed, to get unadulterated PbI2. This may be done by allowing it to react with a hot composition of any bicarbonate. This will result in a cloudy lead carbonate precipitate which then can be easily segregated from Lead Iodide using filtration and we will be left with the golden crystals of Lead Iodide.
Alternatively, one may also produce Lead Iodide by getting iodine vapour to react with molten lead, at a controlled temperature ranging between 500 degrees to 700 degrees celsius.
No matter how spectacular the formation of Lead Iodide may be, it entails several health hazards. This is because any lead-bearing compounds can be highly toxic, especially when heated. Hence, adequate precautions must be taken while chemically preparing Lead Iodide.
Does PbI2 dissolve in water?
Lead iodide (PbI2) is a yellow crystalline solid that is insoluble in water. It is also denser than water. When placed in water, lead iodide establishes the following equilibrium:
PbI2 (s) ↔ Pb2+ (aq) + 2I- (aq)
The solubility of lead iodide in water is 0.44 g/L at 0°C, 0.76 g/L at 20°C, and 4.1 g/L at 100°C.
Lead iodide is also insoluble in other solvents.
Is lead II iodide ionic or covalent?
Lead iodide is an ionic compound. It's made up of a lead cation and an iodide anion. The lead (II) cation has a +2 charge.
Lead iodide is also known as plumbous iodide. It's a bright yellow, heavy powder that's odorless. It's denser than water and is insoluble in most liquids, except for boiling water and potassium iodine.
Lead iodide is used in bronzing, gold pencils, mosaic gold, printing, and photography.
 E-mail: info@chemborun.com
E-mail: info@chemborun.com Tel: +86-574-87178138
Tel: +86-574-87178138  No. 1558, Jiangnan Road,, Ningbo, Zhejiang, China (Mainland)/31
No. 1558, Jiangnan Road,, Ningbo, Zhejiang, China (Mainland)/31