
Sitemap
-
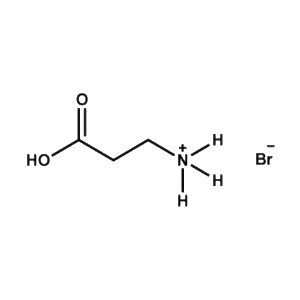
3-APAB (3-Ammonium propionic acid bromide) -
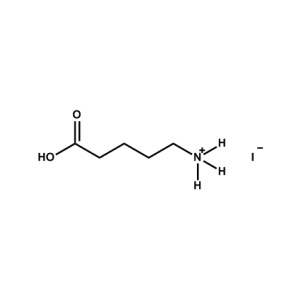
5-AVAI (5-Ammonium valeric acid iodide) -
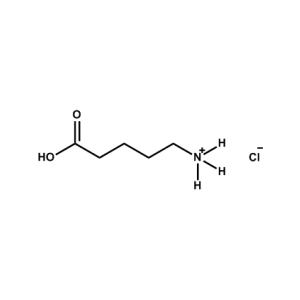
5-AVAC (5-Ammonium valeric acid chloride) -
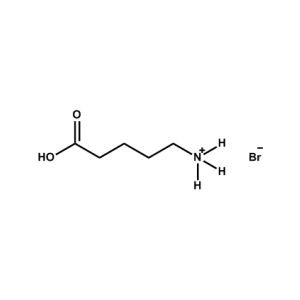
5-AVAB (5-Ammonium valeric acid bromide) -
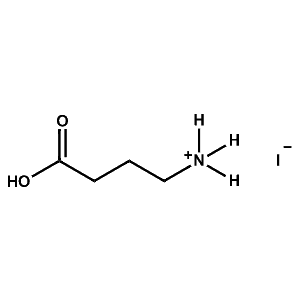
4-ABAI (4-Ammonium butyric acid iodide) -
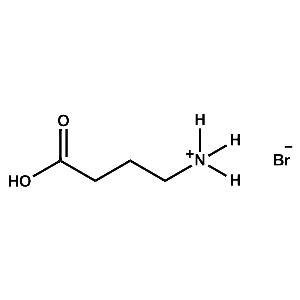
4-ABAB (4-Ammonium butyric acid bromide) -
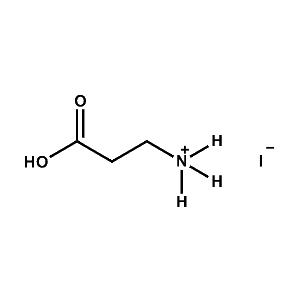
3-APAI (3-Ammonium propionic acid iodide) -

4-bromo-N,N-bis(4-methoxyphenyl)aniline -
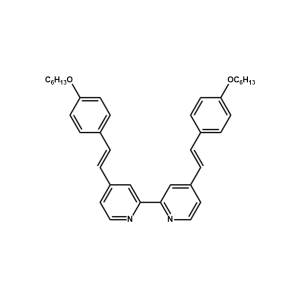
K19 Ligand -
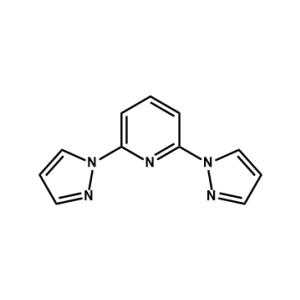
FK 269 Ligand -
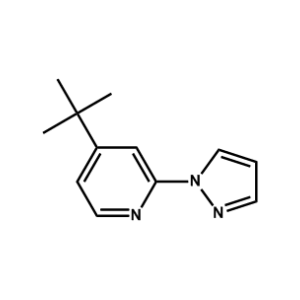
FK 209 Ligand -
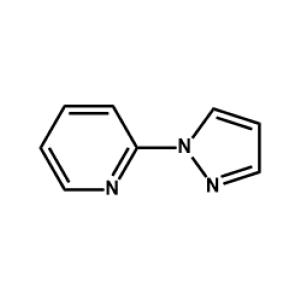
FK 102 Ligand -
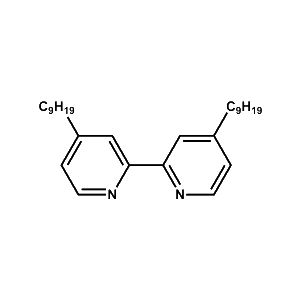
DNBP Hydrophobic Ligand -
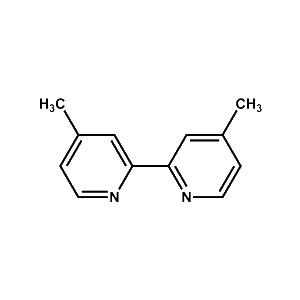
DMBP Building Block Ligand -
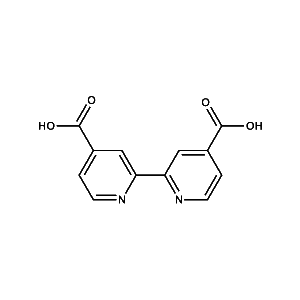
DCBP Anchoring Ligand -

1-Chloro-2,4-bis(hexyloxy)benzene -
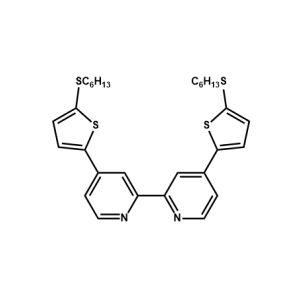
C106 Ligand -
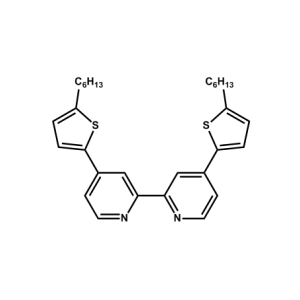
C101 Ligand -

4-Bromo-N,N-bis(4-iodophenyl)aniline -
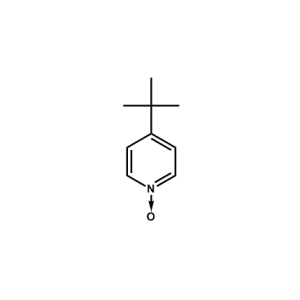
4-(tert-butyl)pyridine-N-oxide -
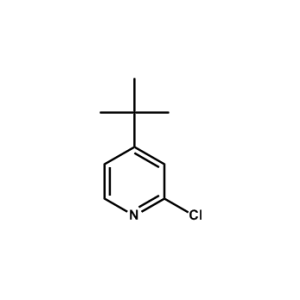
4-(tert-butyl)-2-chloropyridine -

5-Azaspiro[4.4]nonan-5-ium bis(trifluoromethane)sulfonimide -
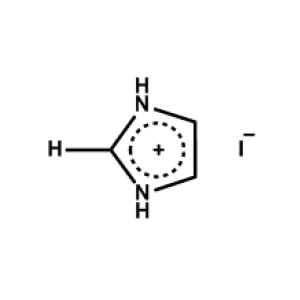
Imidazolium iodide -
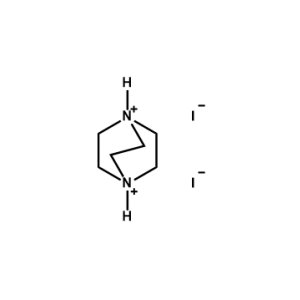
1,4-Diazabicyclo[2,2,2]octane-1,4-diium iodide -
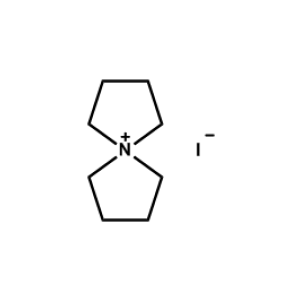
5-Azaspiro[4.4]nonan-5-ium iodide -
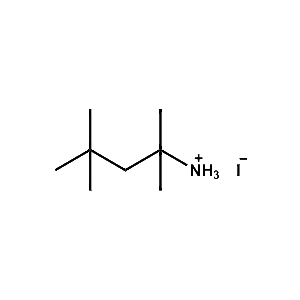
tert-Octylammonium iodide -
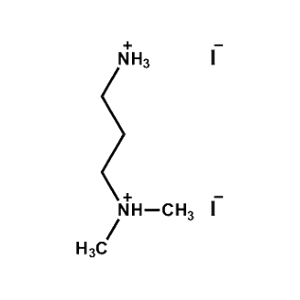
N,N-dimethylpropane- 1,3-diammonium iodide -
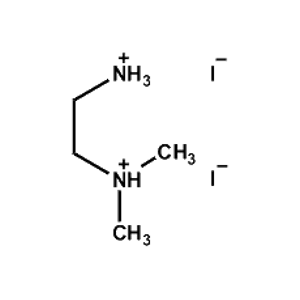
N,N-Dimethylethane- 1,2-diammonium iodide -
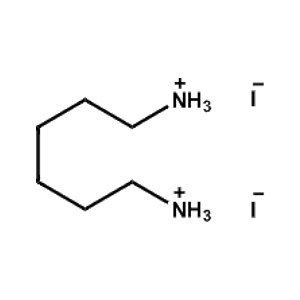
Hexane-1,6-diammonium iodide -
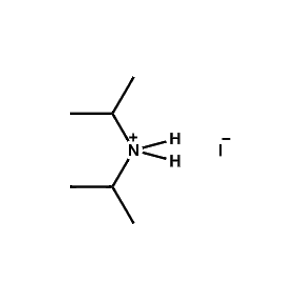
Di-isopropylammonium iodide -
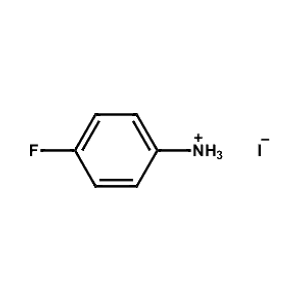
4-Fluoro-Phenylammonium iodide -
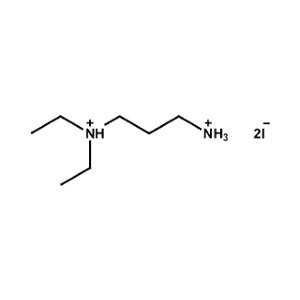
N,N-Diethylpropane-1,3-diammonium iodide -
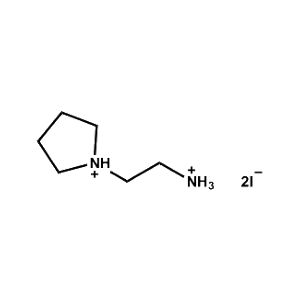
2-Pyrrolidin-1-ium-1-ylethylammonium iodide -
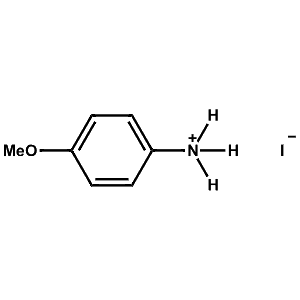
4-Methoxy-Phenylammonium iodide -
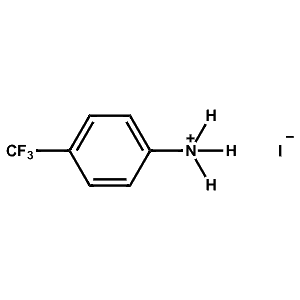
4-Trifluoromethyl-Phenylammonium iodide -
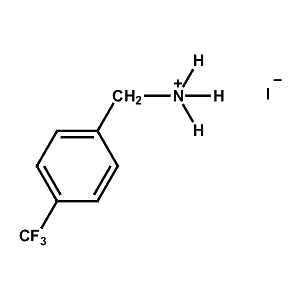
4-Trifluoromethyl-Benzylammonium iodide -
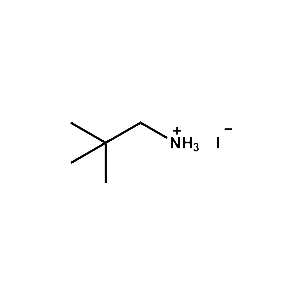
neo-Pentylammonium iodide -
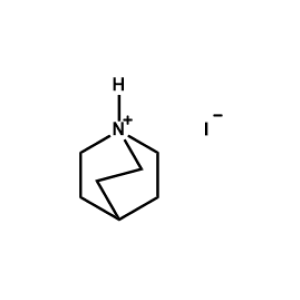
Quinuclidin-1-ium iodide -
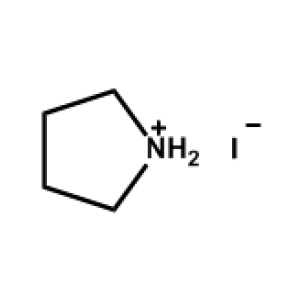
Pyrrolidinium Iodide -
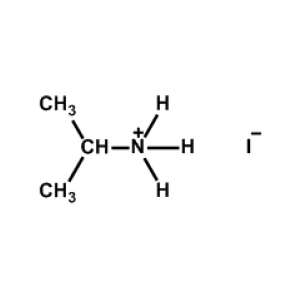
iso-Propylammonium iodide -
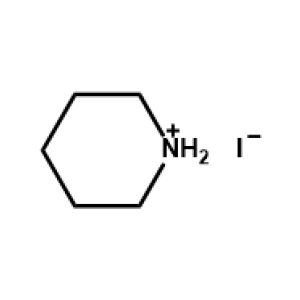
Piperidinium iodide -
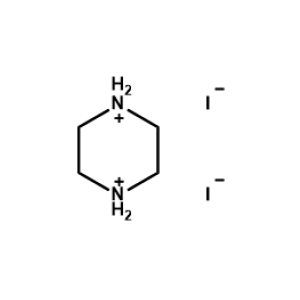
Piperazine-1,4-diium iodide -
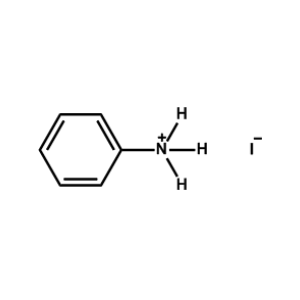
Phenylammonium iodide -
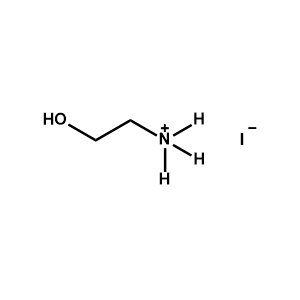
Ethanolammonium iodide -
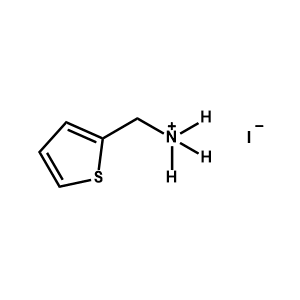
2-Thiophenemethylammonium iodide -
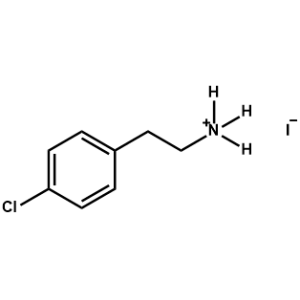
4-Chlorophenethylammonium iodide -

Di-propylammonium iodide -

Diphenylammonium iodide -
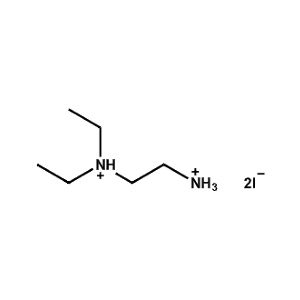
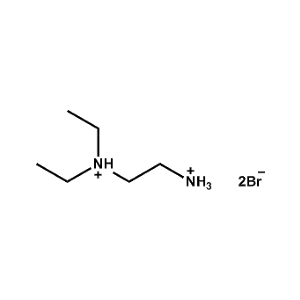
N,N-Diethylethane-1,2-diammonium iodide -
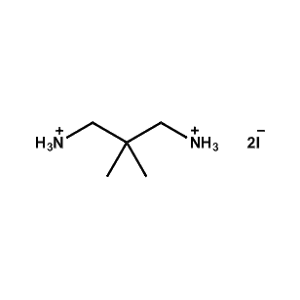
2,2-dimethylpropane-1,3-diammonium iodide -
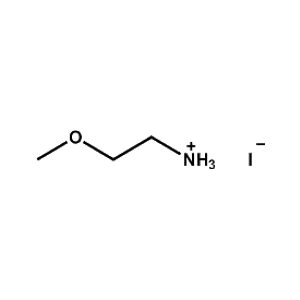
2-Methoxyethylammonium iodide -
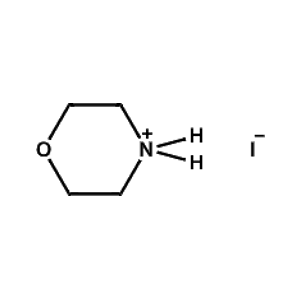
Morpholinium iodide -
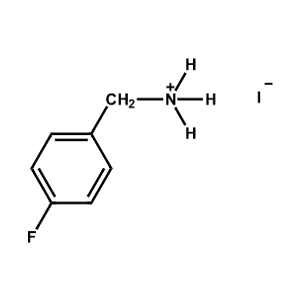
4-Fluoro-Benzylammonium iodide -
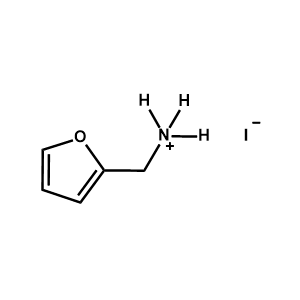
2-Furanemethylammonium iodide -
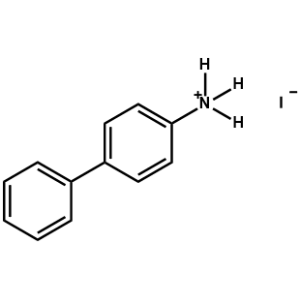
Biphenylammonium iodide -
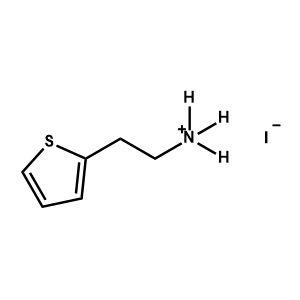
2-Thiopheneethylammonium iodide -
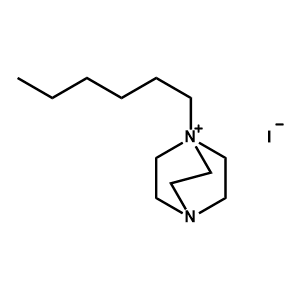
1-n-Hexyl-DABCO iodide -
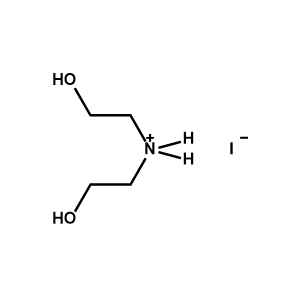
Diethanolammonium iodide -
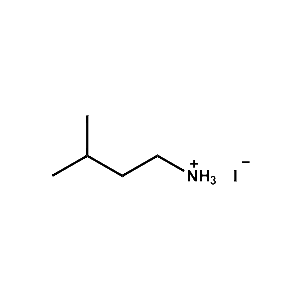
iso-Pentylammonium iodide -
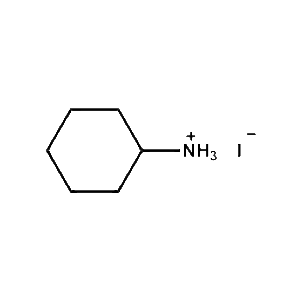
Cyclohexylammonium iodide -
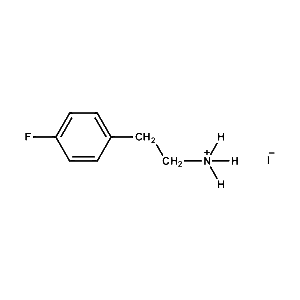
4-Fluoro-Phenethylammonium iodide -
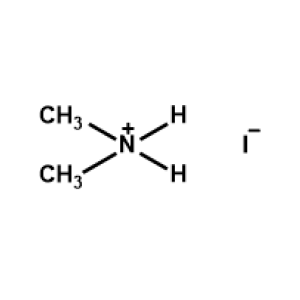
Dimethylammonium iodide -
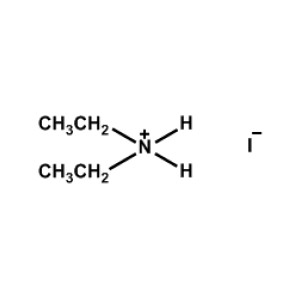
Diethylammonium iodide -
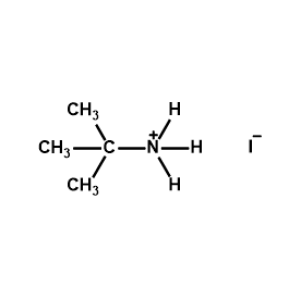
t-Butylammonium iodide -
n-Butylammonium iodide -
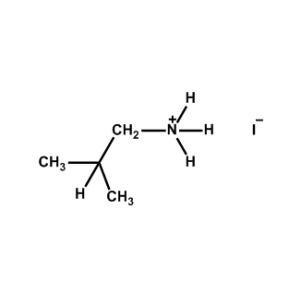
iso-Butylammonium iodide -
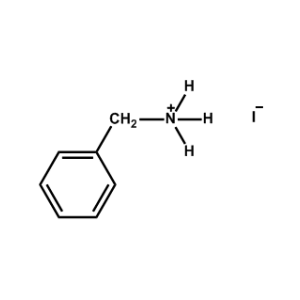
Benzylammonium iodide -
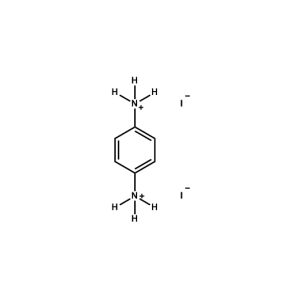
1,4-Benzene diammonium iodide -
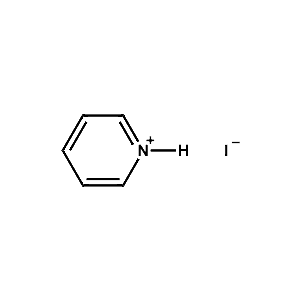
Pyridinium iodide -
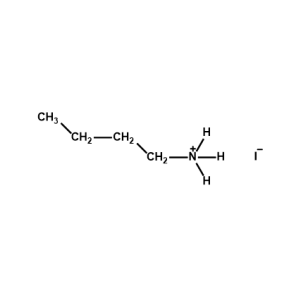
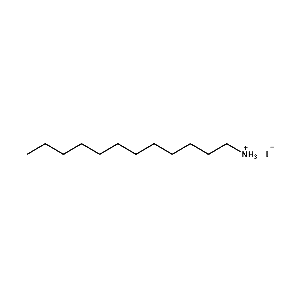
n-Dodecylammonium iodide -
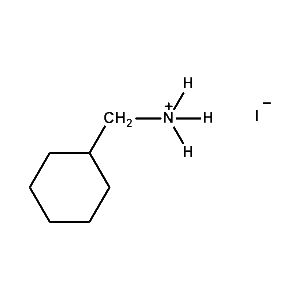
Cyclohexylmethylammonium iodide -
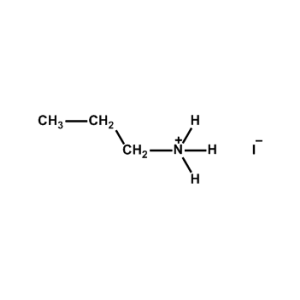
n-Propylammonium iodide -
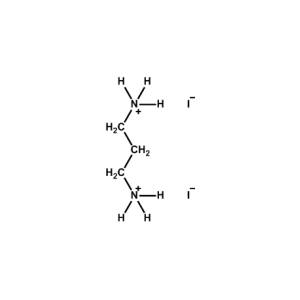
Propane-1,3-diammonium iodide -
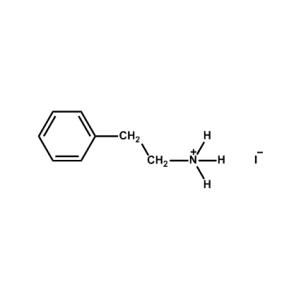
Phenethylammonium iodide -
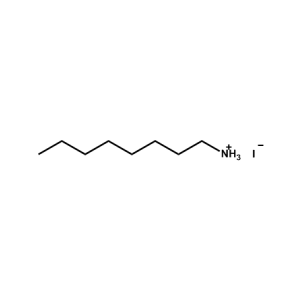
n-Octylammonium Iodide -
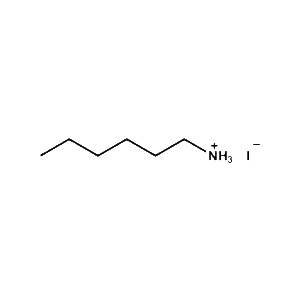
n-Hexylammonium iodide -
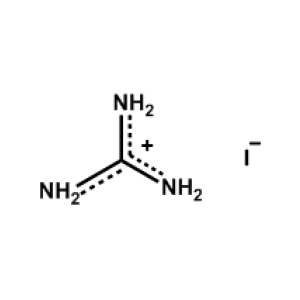
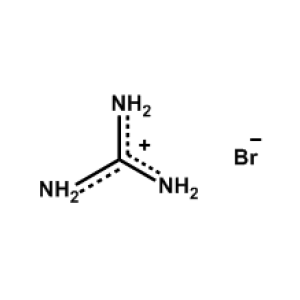
Guanidinium iodide -
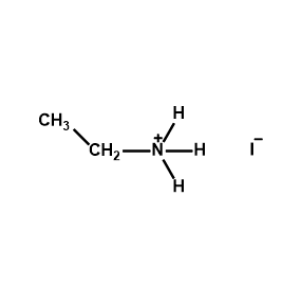
Ethylammonium iodide -
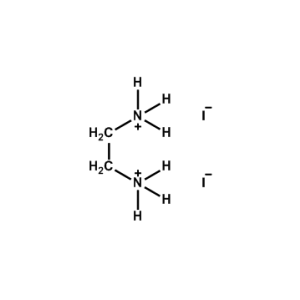
Ethane-1,2-diammonium iodide -
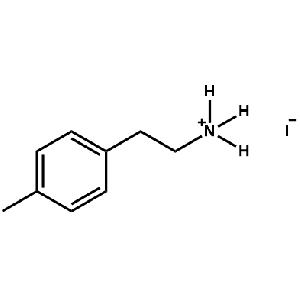
4-Methylphenethylammonium iodide -
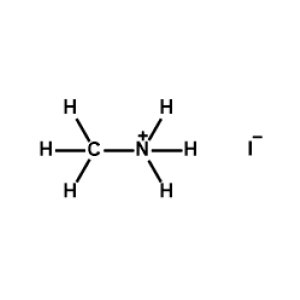
Methylammonium iodide >99.99%, CAS 14965-49-2 -
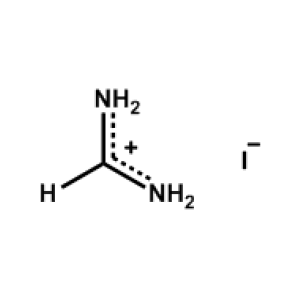
Formamidinium iodide >99.99%, CAS 879643-71-7 -
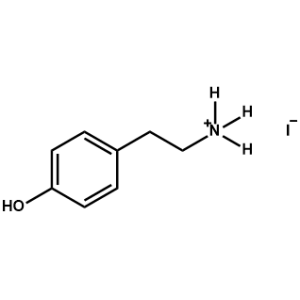
4Hydroxyphenethylammonium iodide -
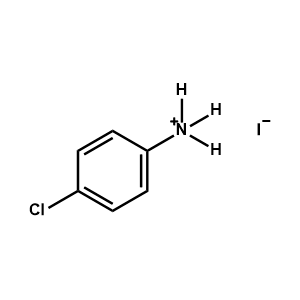
4-Chlorophenylammonium iodide -
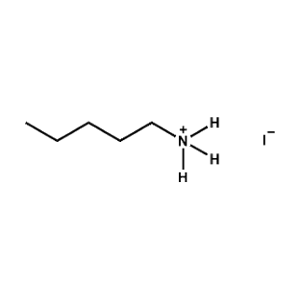
n-Pentylammonium iodide -
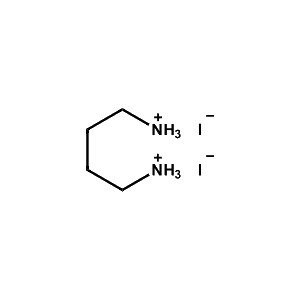
Butane-1,4-diammonium iodide -
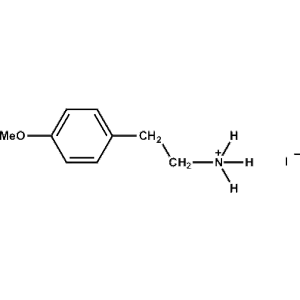
4-Methoxy-Phenethylammonium iodide -
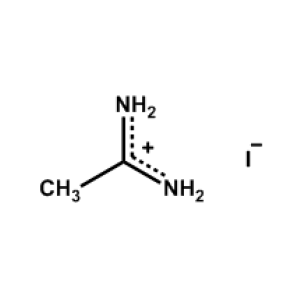
Acetamidinium iodide -
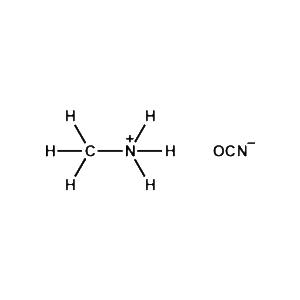
Methylammonium cyanate -
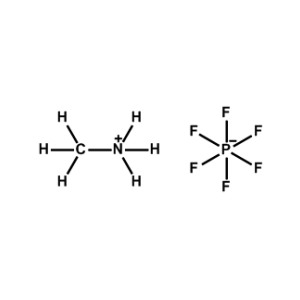
Methylammonium hexafluorophosphate -
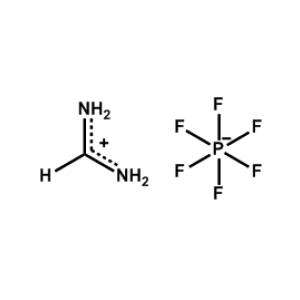
Formamidinium hexafluorophosphate -
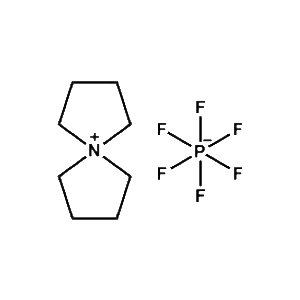
5-Azaspiro[4.4]nonan-5-ium hexafluorophosphate -

n-Butylammonium acetate -
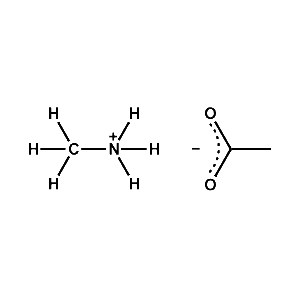
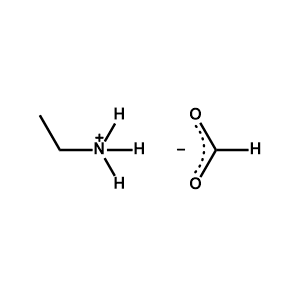
Ethylammonium formate -
Methylammonium acetate -
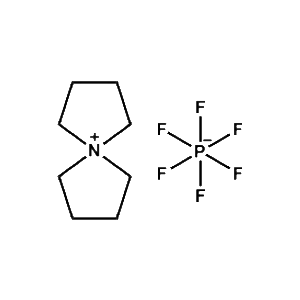
5-Azaspiro[4.4]nonan-5-ium tetrafluoroborate -
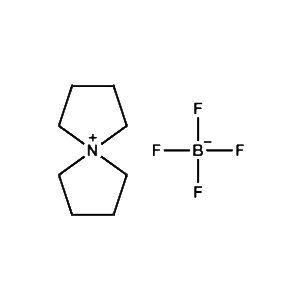
5-Azaspiro[4.4]nonan-5-ium iodide -
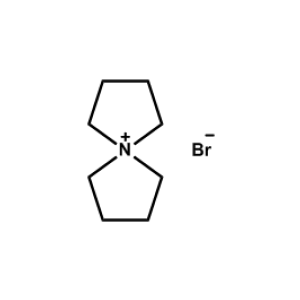
5-Azaspiro[4.4]nonan-5-ium hexafluorophosphate -
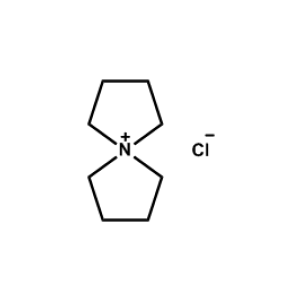
5-Azaspiro[4.4]nonan-5-ium chloride -
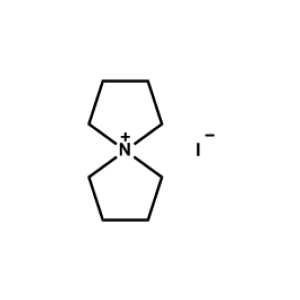
5-Azaspiro[4.4]nonan-5-ium bromide -

5-Azaspiro[4.4]nonan-5-ium bis(trifluoromethane)sulfonimide -
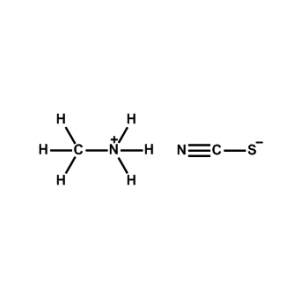
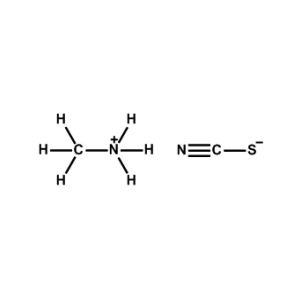
Methylammonium thiocyanate -
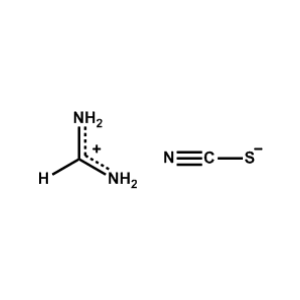
Formamidinium thiocyanate -
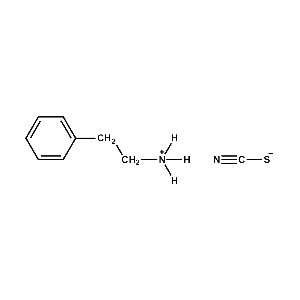
Phenethylammonium thiocyanate -
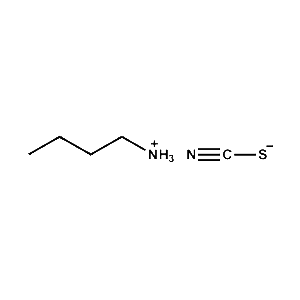
n-Butylammonium thiocyanate -
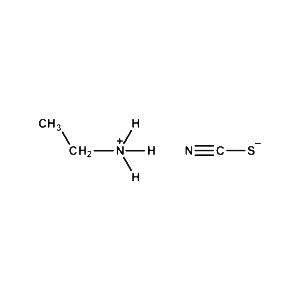
Ethylammonium thiocyanate -
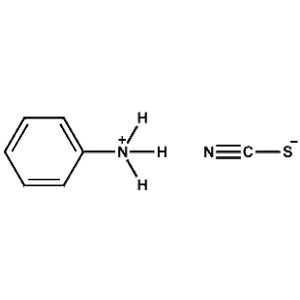
Phenylammonium thiocyanate -
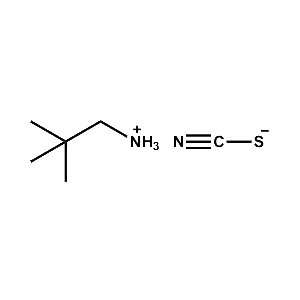
neo-Pentylammonium thiocyanate -
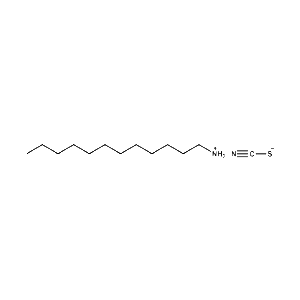
n-Dodecylammonium thiocyanate -
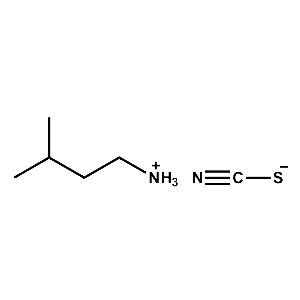
i-Pentylammonium thiocyanate -
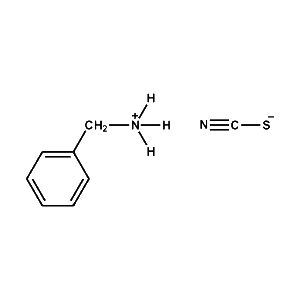
Benzylammonium thiocyanate -
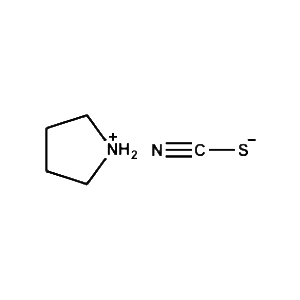
Pyrrolidinium thiocyanate -
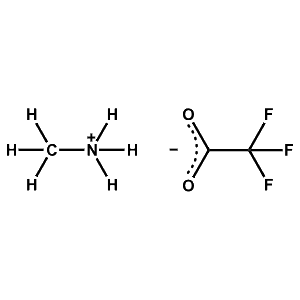
Methylammonium trifluoroacetate -
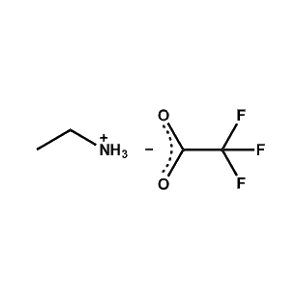
Ethylammonium trifluoroacetate -
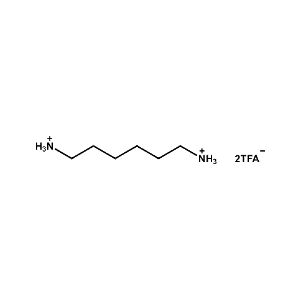
Hexane-1,6-diammonium trifluoroacetate -
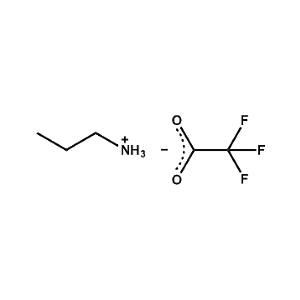
n-Propylammonium trifluoroacetate -

Di-propylammonium trifluoroacetate -

Diphenylammonium trifluoroacetate -

Di-Methylammonium trifluoroacetate -

Di-butylammonium trifluoroacetate -

Butane-1,4-diammonium trifluoroacetate -
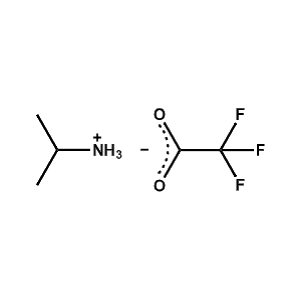
iso-Propylammonium trifluoroacetate -
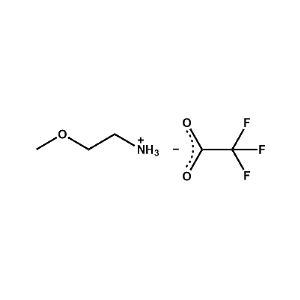
2-Methoxyethylammonium trifluoroacetate -
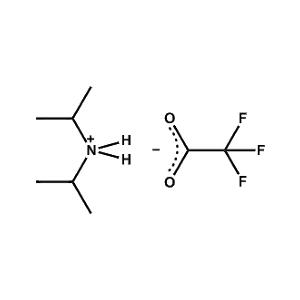
Di-isopropylammonium trifluoroacetate -
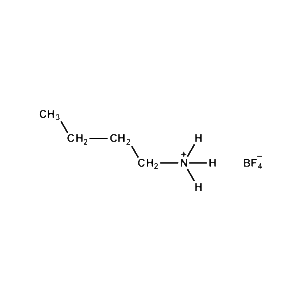
n-Butylammonium tetrafluoroborate -
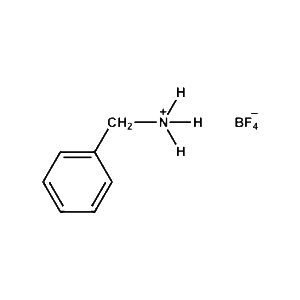
Benzylammonium tetrafluoroborate -
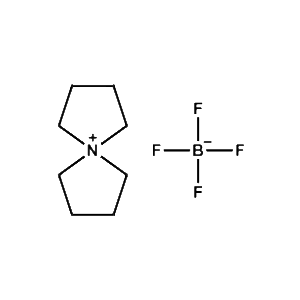
5-Azaspiro[4.4]nonan-5-ium tetrafluoroborate -
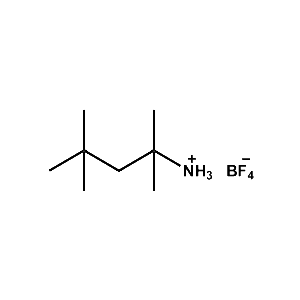
tert-Octylammonium tetrafluoroborate -
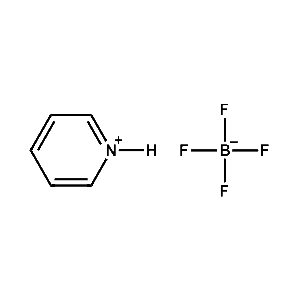
Pyridinium tetrafluoroborate -
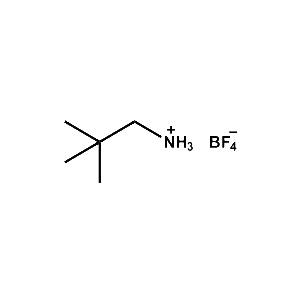
neo-Pentylammonium tetrafluoroborate -
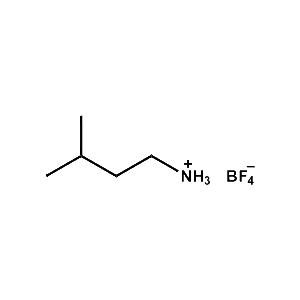
i-Pentylammonium tetrafluoroborate -
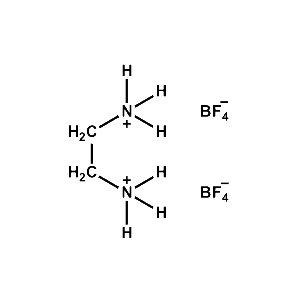
Ethane-1,2- diammonium tetrafluoroborate -
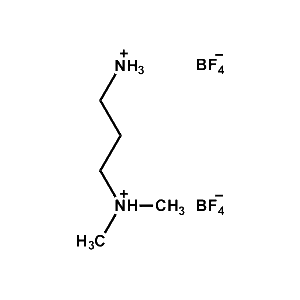
N,N-dimethylpropane- 1,3-diammonium tetrafluoroborate -
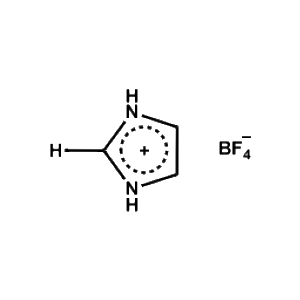
Imidazolium tetrafluoroborate -
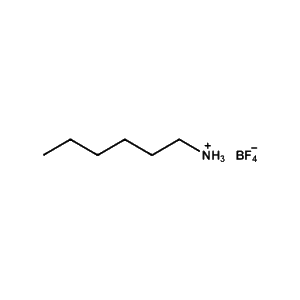
n-Hexylammonium tetrafluoroborate -
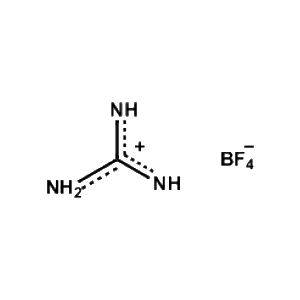
Guanidinium tetrafluoroborate -
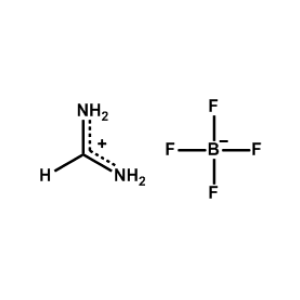
Formamidinium tetrafluoroborate -
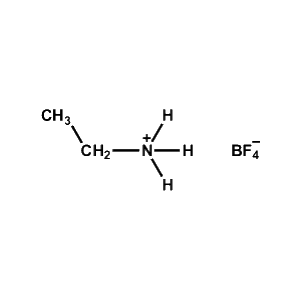
Ethylammonium tetrafluoroborate -
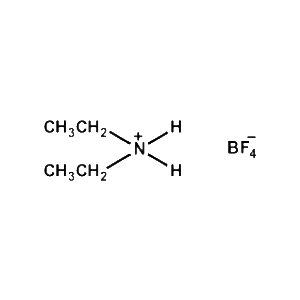
Diethylammonium tetrafluoroborate -
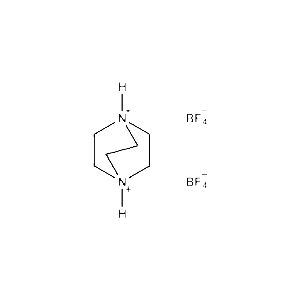
1,4-Diazabicyclo[2,2,2]octane-1,4-diium tetrafluoroborate -
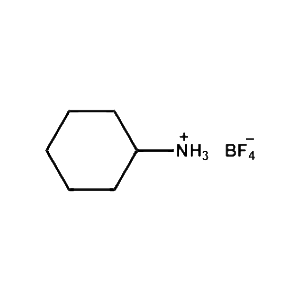
Cyclohexylammonium tetrafluoroborate -
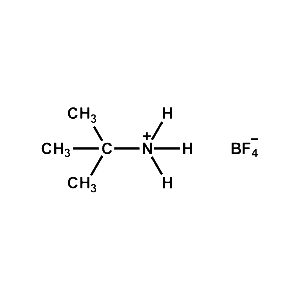
t-Butylammonium tetrafluoroborate -
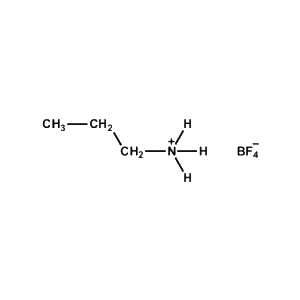
n-Propylammonium tetrafluoroborate -
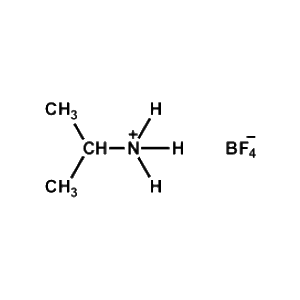
iso-Propylammonium tetrafluoroborate -
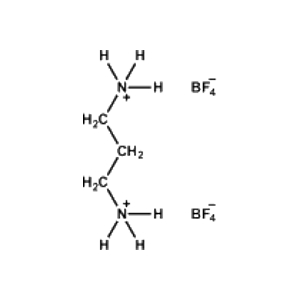
Propane-1,3-diammonium tetrafluoroborate -
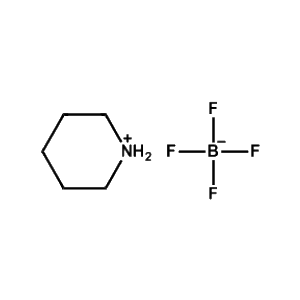
Piperidinium tetrafluoroborate -
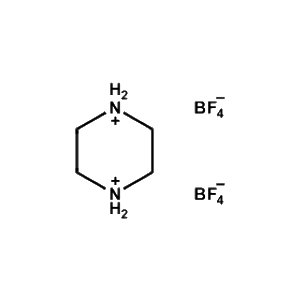
Piperazine-1,4-diium tetrafluoroborate -
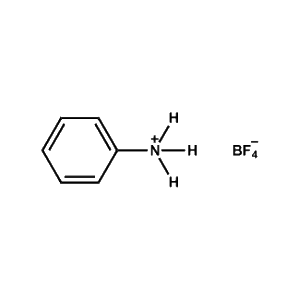
Phenylammonium tetrafluoroborate -
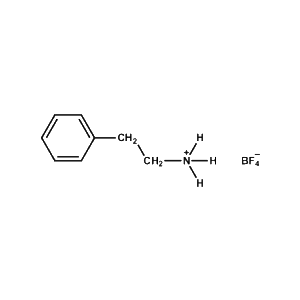
Phenethylammonium tetrafluoroborate -
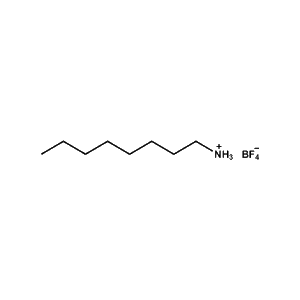
n-Octylammonium tetrafluoroborate -
Methylammonium tetrafluoroborate -
N,N-Diethylethane-1,2-diammonium tetrafluoroborate -
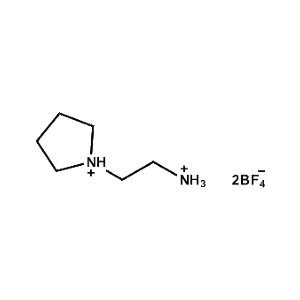
2-Pyrrolidin-1-ium-1-ylethylammonium tetrafluoroborate -
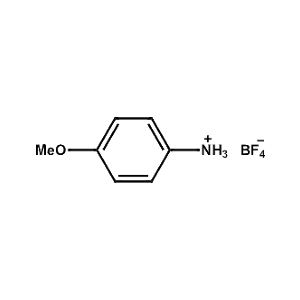
4-Methoxy-Phenylammonium tetrafluoroborate -
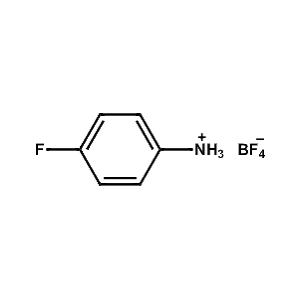
4-Fluoro-Phenylammonium tetrafluoroborate -
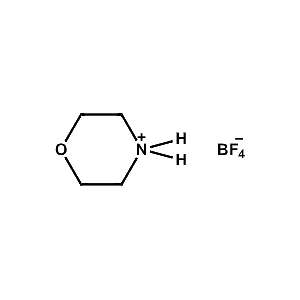
Morpholinium tetrafluoroborate -
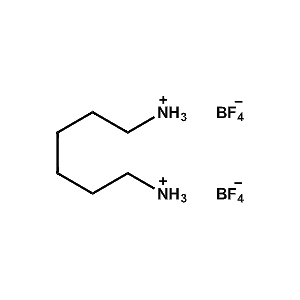
Hexane-1,6-diammonium tetrafluoroborate -
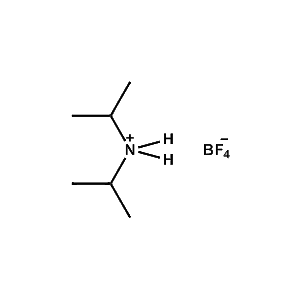
Di-iso-Propylammonium tetrafluoroborate -
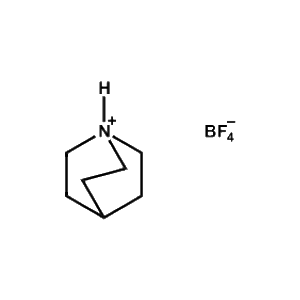
Quinuclidin-1-ium tetrafluoroborate -
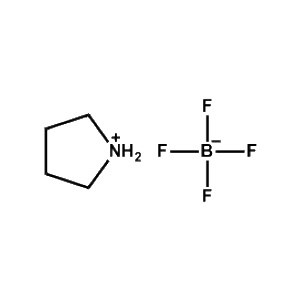
Pyrrolidinium tetrafluoroborate -
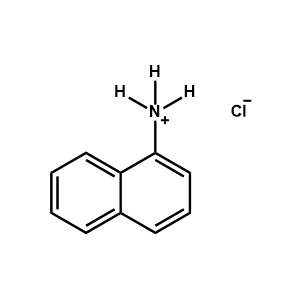
1-Naphthylammonium chloride -
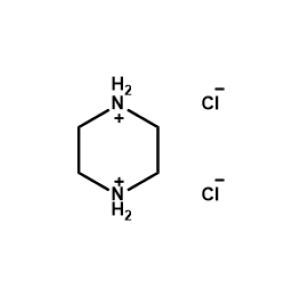
Piperazine-1,4-diium chloride -
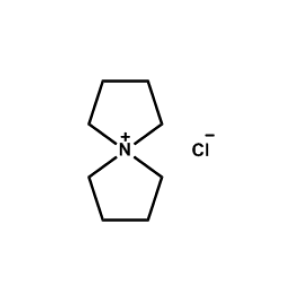
5-Azaspiro[4.4]nonan-5-ium chloride -
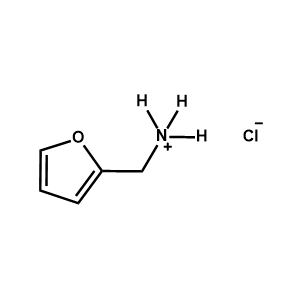
2-Furanemethylammonium chloride -
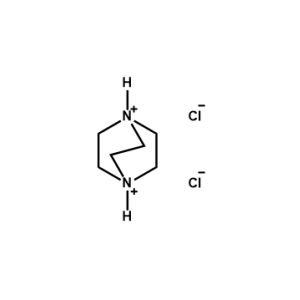
1,4-Diazabicyclo[2.2.2]octane-1,4-diium chloride
-
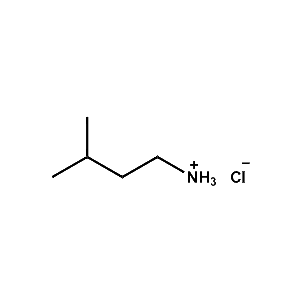
i-Pentylammonium chloride -
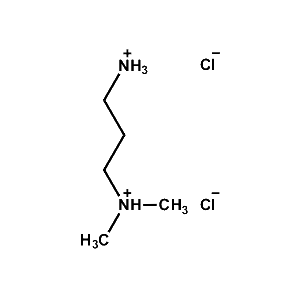
N,N-Dimethylpropane- 1,3-diammonium chloride -
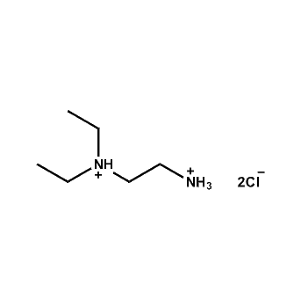
N,N-Diethylethane-1,2-diammonium chloride -
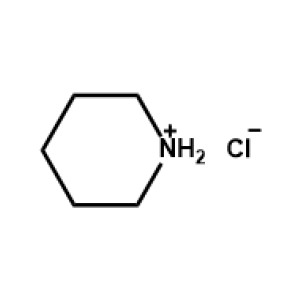
Piperidinium chloride -
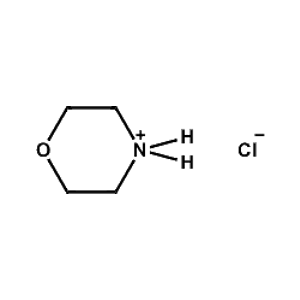
Morpholinium chloride -
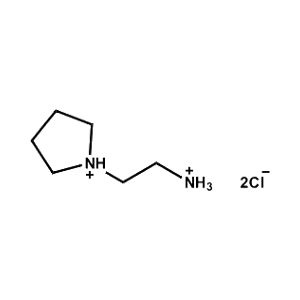
2-Pyrrolidin-1-ium-1-ylethylammonium chloride -
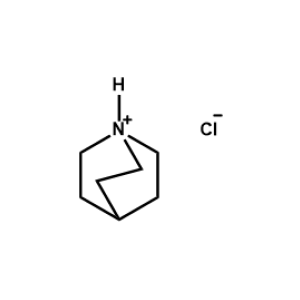
Quinuclidin-1-ium chloride -
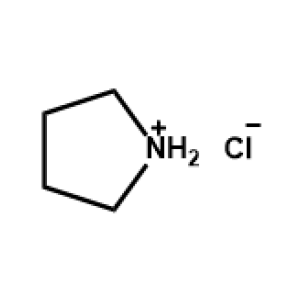
Pyrrolidinium chloride -
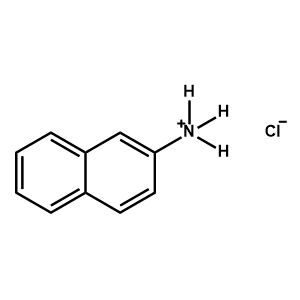
2-Naphthylammonium chloride -
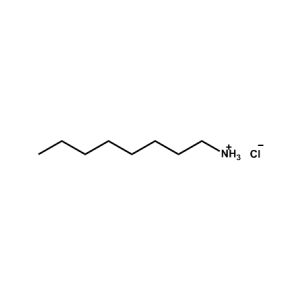
n-Octylammonium Chloride -
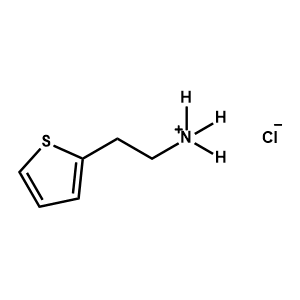
2Thiopheneethylammonium chloride -
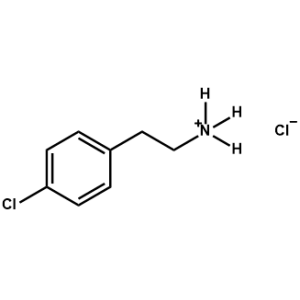
4-Chloro-Phenethylammonium chloride -

Di-Propylammonium chloride -
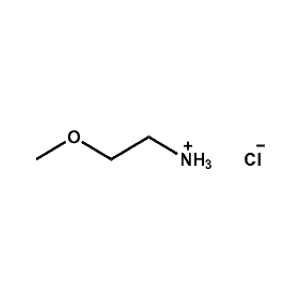
2-Methoxyethylammonium chloride -
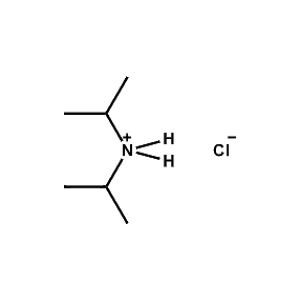
Di-isopropylammonium chloride -
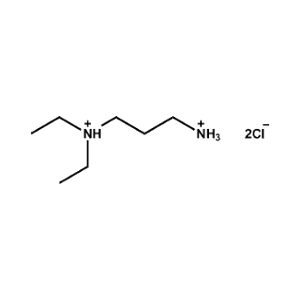
N,N-Diethylpropane-1,3-diammonium chloride -
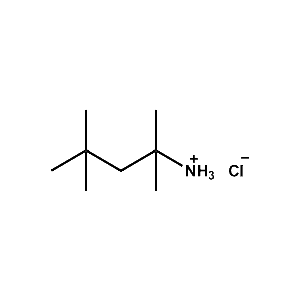
tert-Octylammonium chloride -
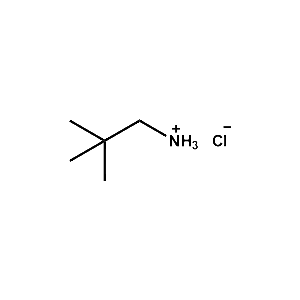
neo-Pentylammonium chloride -
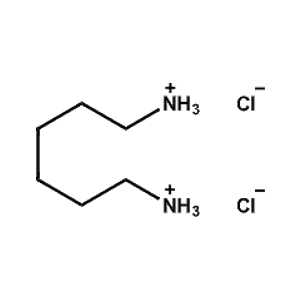
Hexane-1,6-diammonium chloride -
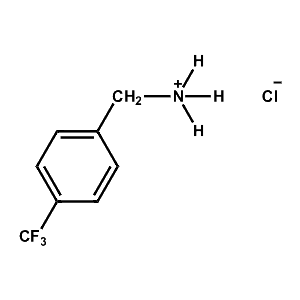
4-Fluoro-Phenylammonium chloride -
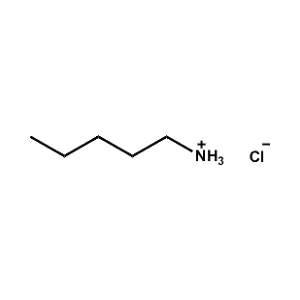
n-Pentylammonium Chloride -
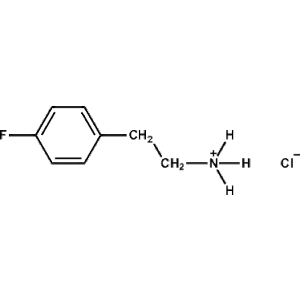
4-Methoxy-Phenethylammonium chloride -
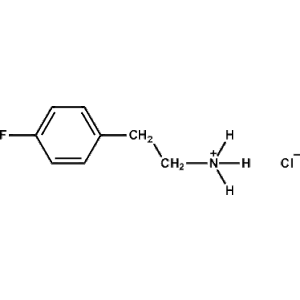
4-Fluoro-Phenethylammonium chloride -
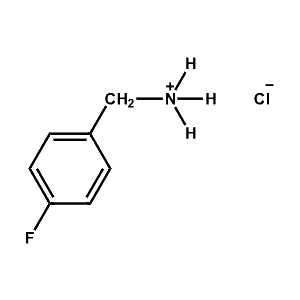
4-Fluoro-Benzylammonium chloride -
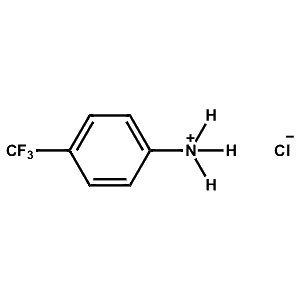
4-Trifluoromethyl-Phenylammonium chloride -
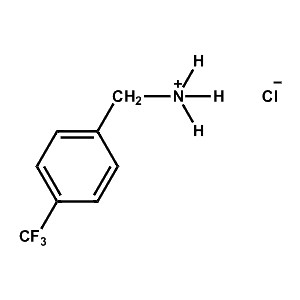
4-Trifluoromethyl-Benzylammonium chloride -
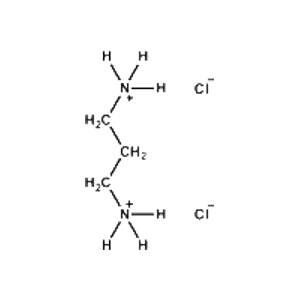
Propane-1,3-diammonium chloride -
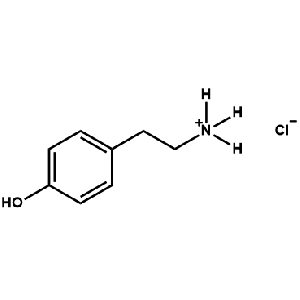
4Hydroxyphenethylammonium chloride -
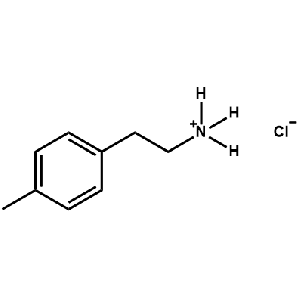
4-Methylphenethylammonium chloride -
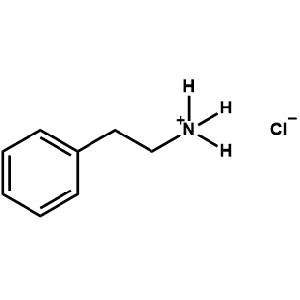
Phenethylammonium chloride -
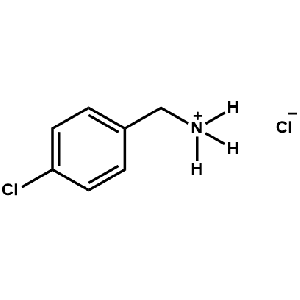
4-Chlorobenzylammonium chloride -
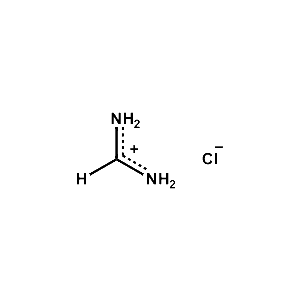
Formamidinium chloride >99.99% -
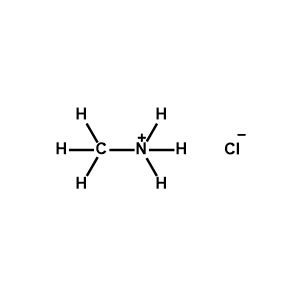
Methylammonium chloride >99.99% -
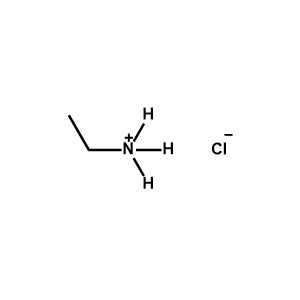
Ethylammonium chloride -

Diphenylammonium chloride -

Di-Butylammonium chloride -
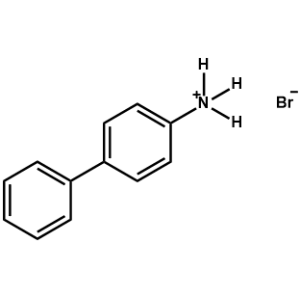
Biphenylammonium bromide -
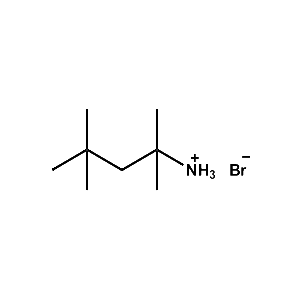
Trimethylammonium bromide -
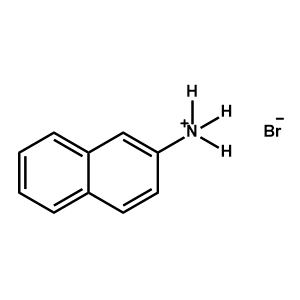
2-Naphthylammonium bromide -
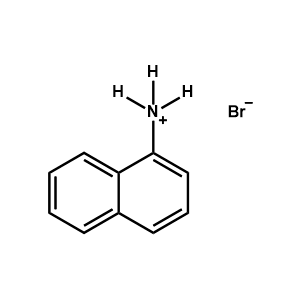
1-Naphthylammonium bromide -
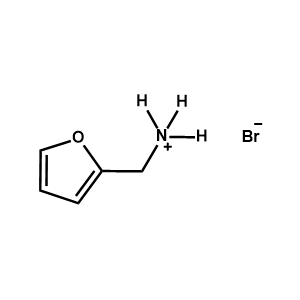
2-Furanemethylammonium bromide -
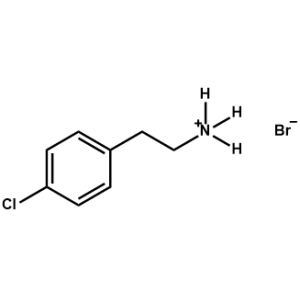
4-Chlorophenethylammonium bromide -

Di-Propylammonium bromide -

Diphenylammonium bromide -

Di-Butylammonium bromide -
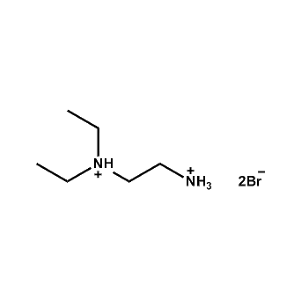
N,N-Diethylethane-1,2-diammonium bromide -
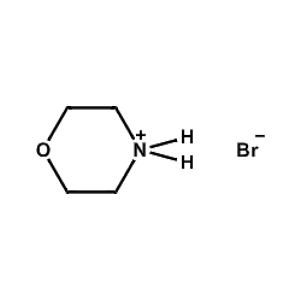
Morpholinium bromide -
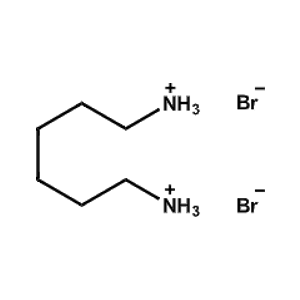
Hexane-1,6-diammonium bromide -
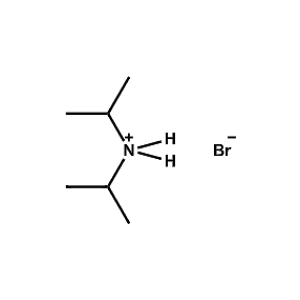
Di-iso-Propylammonium bromide -
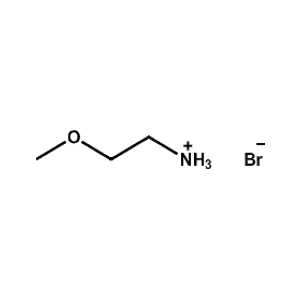
2-Methoxyethylammonium bromide -
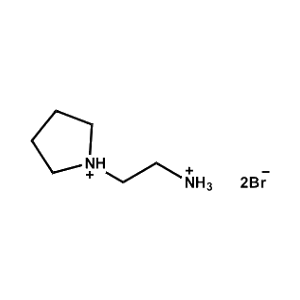
2-Pyrrolidin-1-ium-1-ylethylammonium bromide -
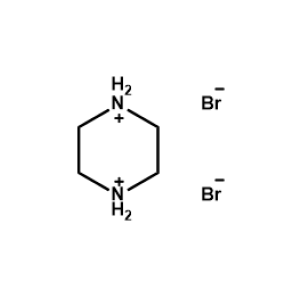
Piperazine-1,4-diium bromide -
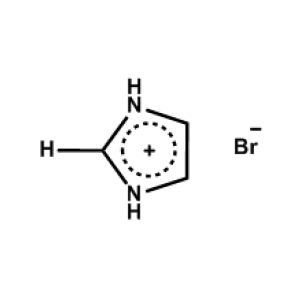
Imidazolium bromide -
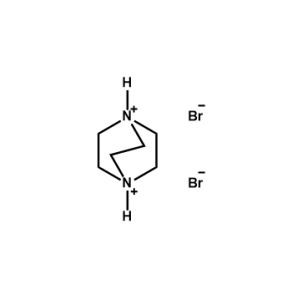
1,4-Diazabicyclo[2,2,2]octane-1,4-diium bromide -
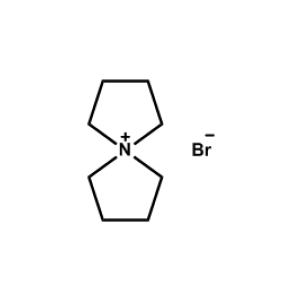
5-Azaspiro[4.4]nonan-5-ium bromide -
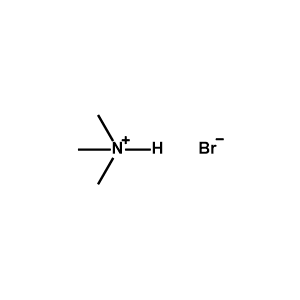
tert-Octylammonium bromide -
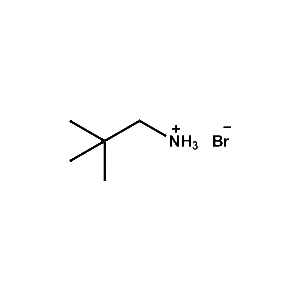
neo-Pentylammonium bromide -
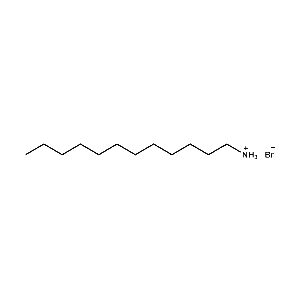
n-Dodecylammonium bromide -
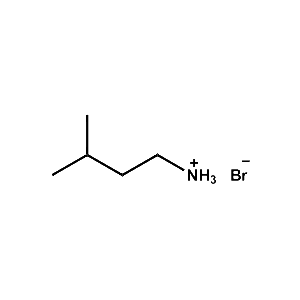
i-Pentylammonium bromide -
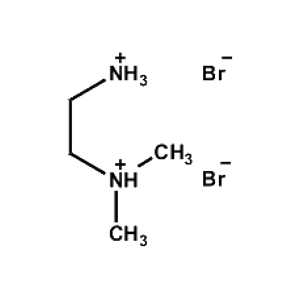
N,N-Dimethylethane- 1,2-diammonium bromide -
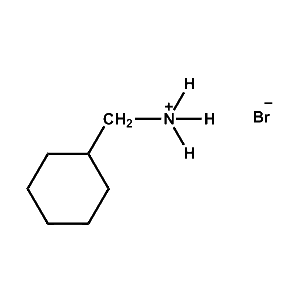
Cyclohexylmethylammonium bromide -
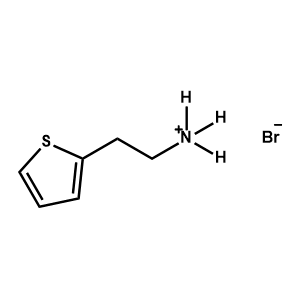
2-Thiopheneethylammonium bromide -
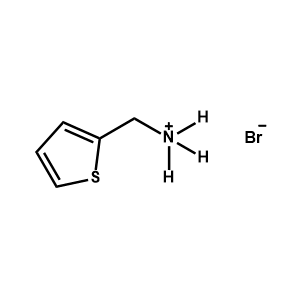
2-Thiophenemethylammonium bromide -
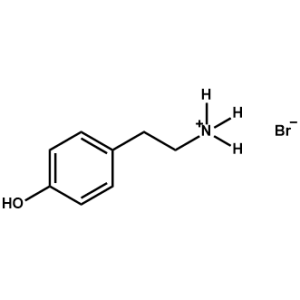
4-Hydroxyphenethylammonium bromide -
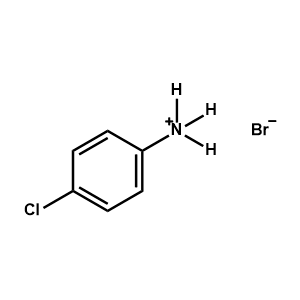
4-Chlorophenylammonium bromide -
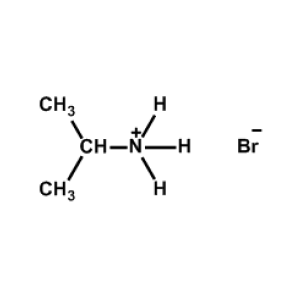
iso-Propylammonium bromide -
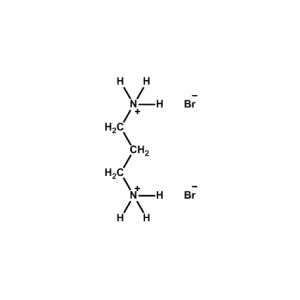
Propane-1,3-diammonium bromide -
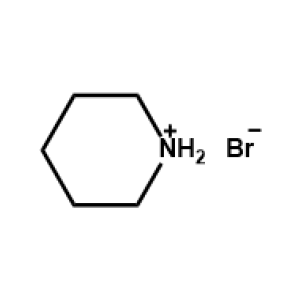
Piperidinium bromide -
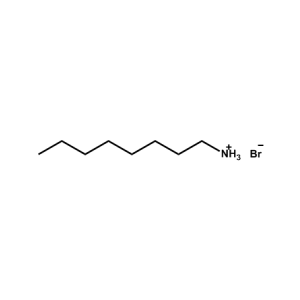
n-Octylammonium Bromide -
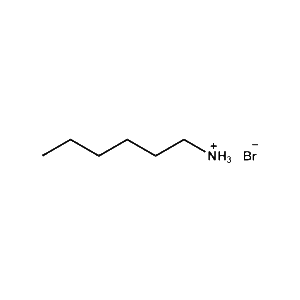
n-Hexylammonium bromide -
Guanidinium bromide -
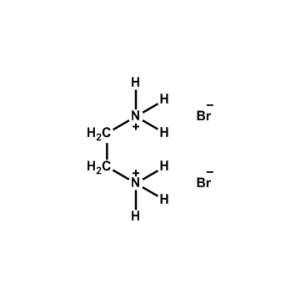
Ethane-1,2-diammonium bromide -
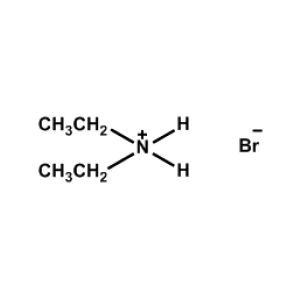
Diethylammonium bromide -
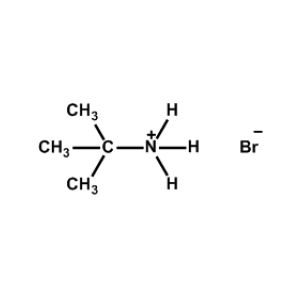
t-Butylammonium bromide -
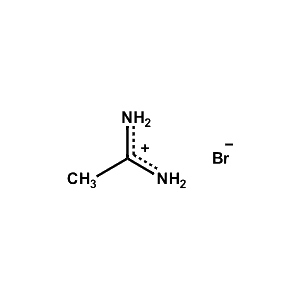
Acetamidinium bromide -
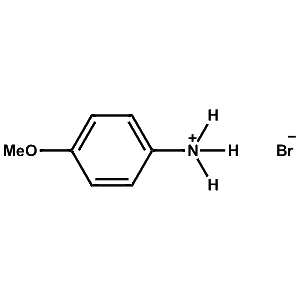
4-Methoxy-Phenylammonium bromide -
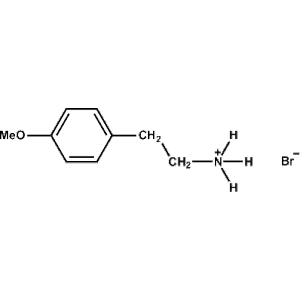
4-Methoxy-Phenethylammonium bromide -
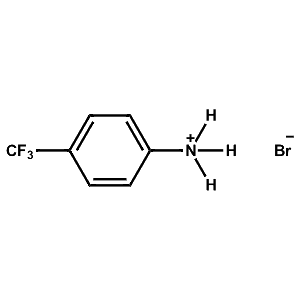
4-Trifluoromethyl-Phenylammonium bromide -
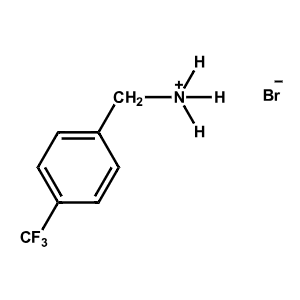
4-Trifluoromethyl-Benzylammonium bromide -
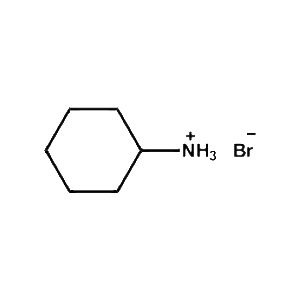
Cyclohexylammonium bromide -
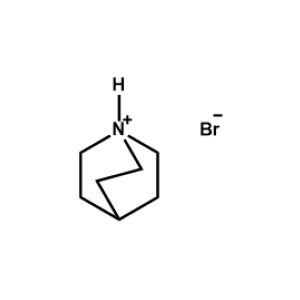
Quinuclidin-1-ium bromide -
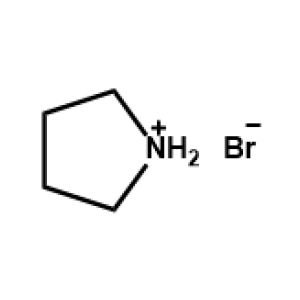
Pyrrolidinium bromide -
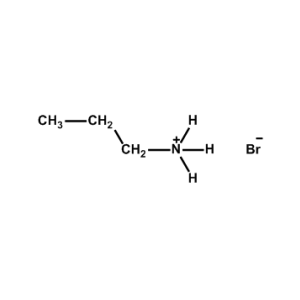
n-Propylammonium bromide -
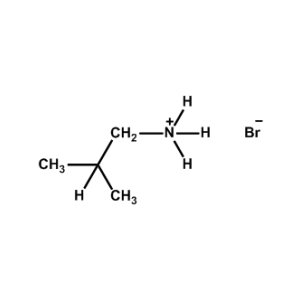
iso-Butylammonium bromide -
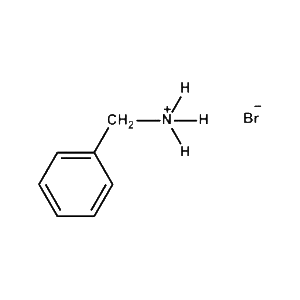
Benzylammonium bromide -
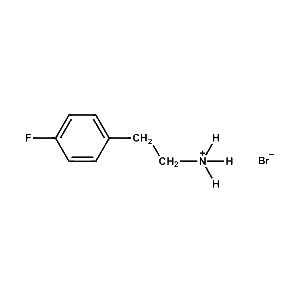
4-Fluoro-Phenethylammonium bromide -
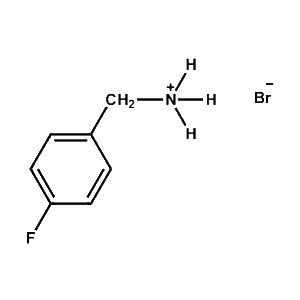
4-Fluoro-Benzylammonium bromide -
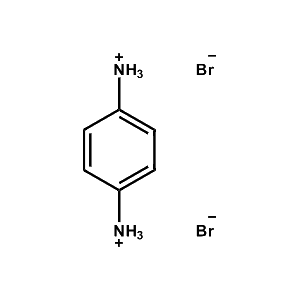
1,4-Benzene diammonium bromide -
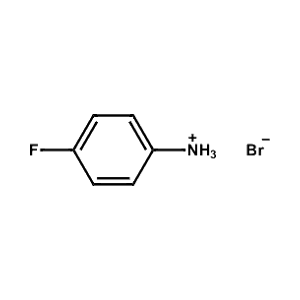
4-Fluoro-Phenylammonium bromide -
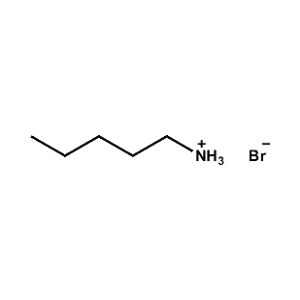
n-Pentylammonium Bromide -
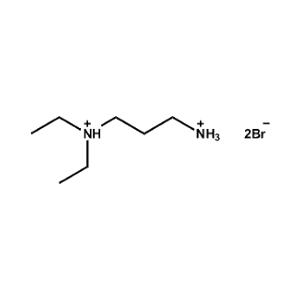
N,N-Diethylpropane-1,3-diammonium bromide -
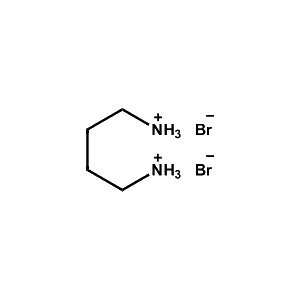
Butane-1,4-diammonium bromide -
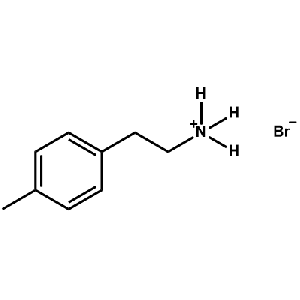
4-Methylphenethylammonium bromide -
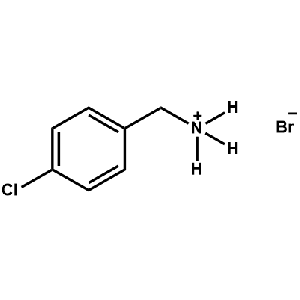
4-Chlorobenzylammonium bromide -
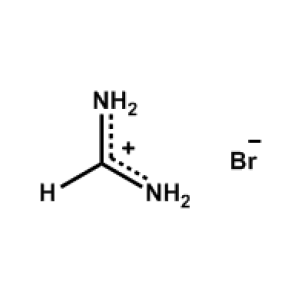
Formamidinium bromide >99.99%, CAS 146958-06-7 -
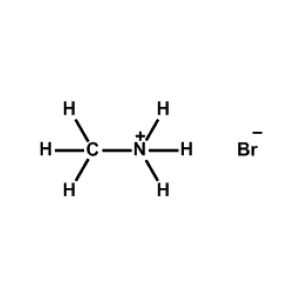
Methylammonium bromide >99.99%, CAS 6876-37-5 -
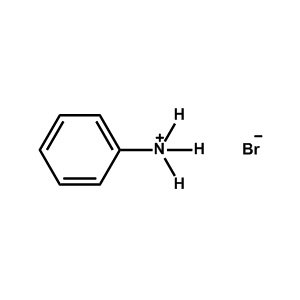
Phenylammonium bromide -
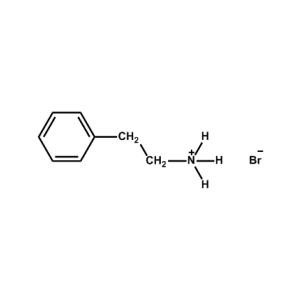
Phenethylammonium bromide -
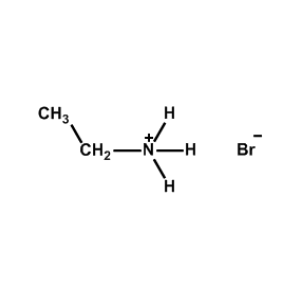
Ethylammonium bromide -
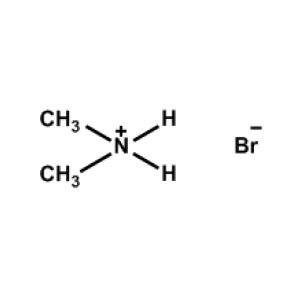
Dimethylammonium bromide -
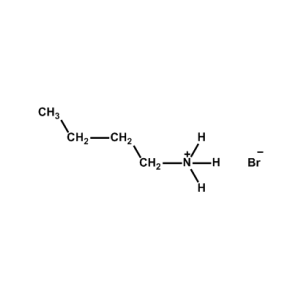
n-Butylammonium bromide -
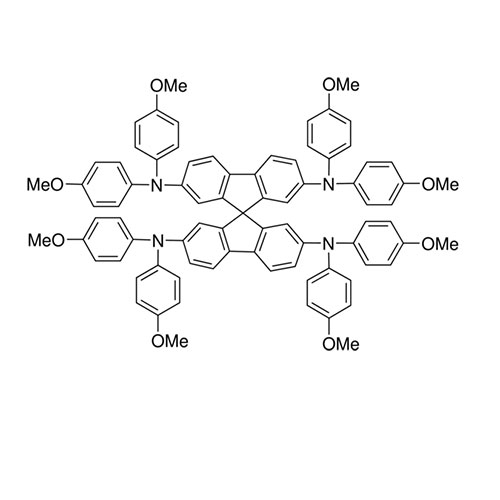
Spiro-OMeTAD (spiro-ometad) sublimed(99.8% and 99.9% purity) -
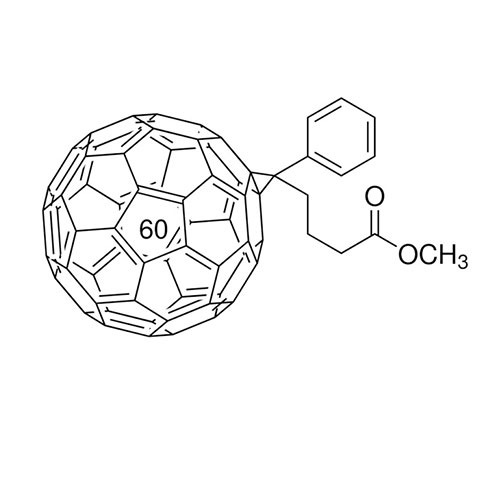
[6,6]-Phenyl C61 butyric acid methyl ester >99.5% Spiro-OMeTAD -

Spiro-OMeTAD C60 -
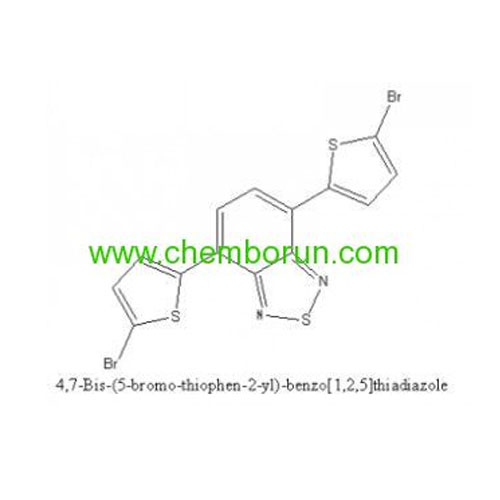
4,7-Bis(5-bromothiophen-2-yl)-5,6-difluorobenzo[c][1,2,5] thiadiazole -
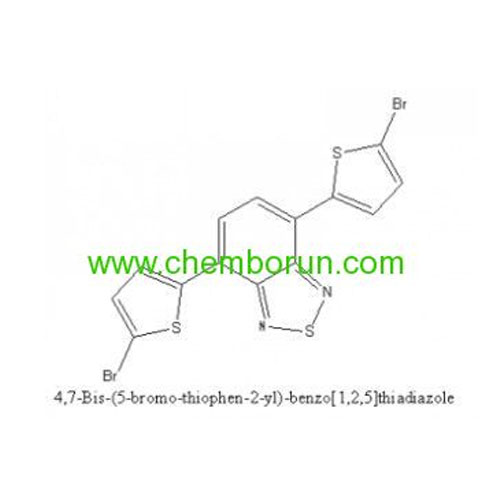
4,7-Bis(2-bromo-5-thienyl)-2,1,3-benzothiadiazole -
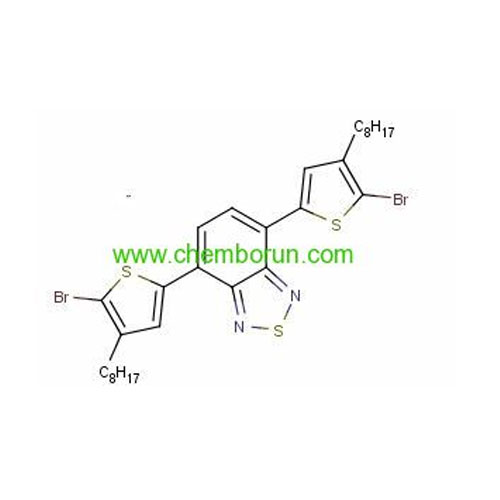
4,7-Bis(5-bromo-4-octylthiophen-2-yl)benzo[c][1,2,5]thiadiazole -
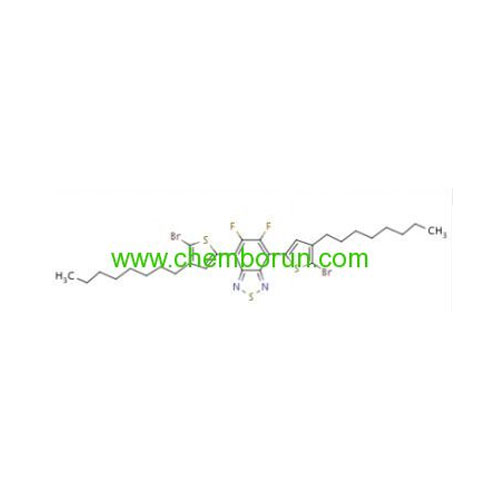
4,7-bis(5-bromo-4-octylthiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole -
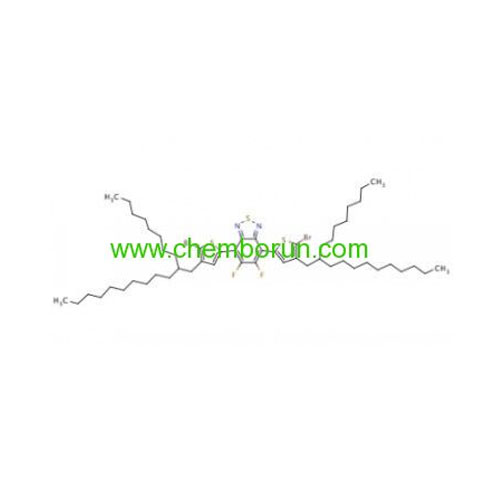
5,6-difluoro-4,7-bis[5-bromo-4-(2-octyldodecyl)thiophene-2-yl]benzo[c][1,2,5]thiadiazole -
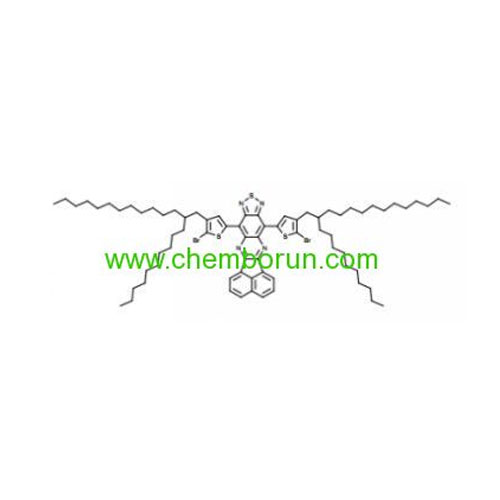
PBDTTQ-3 and PAPhTQ -
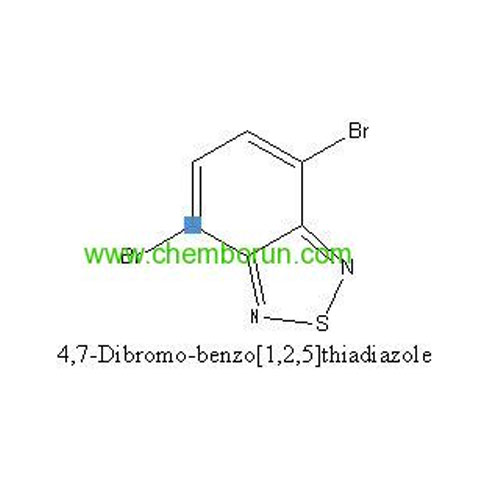
98% 4,7-Dibromobenzo[c][1,2,5]thiadiazole -
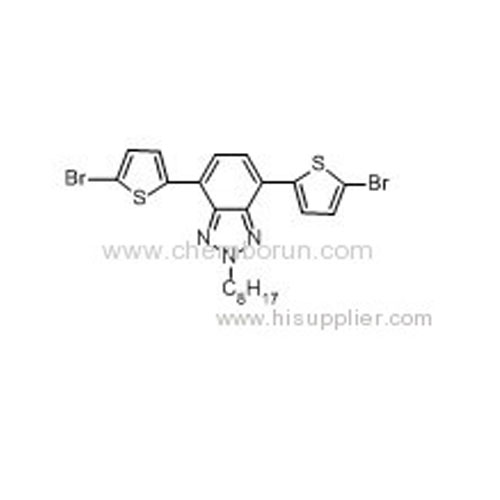
2-Octyl-4,7-di(5-bromo-thiophen-2-yl)-2H-benzo[d][1,2,3]triazole -
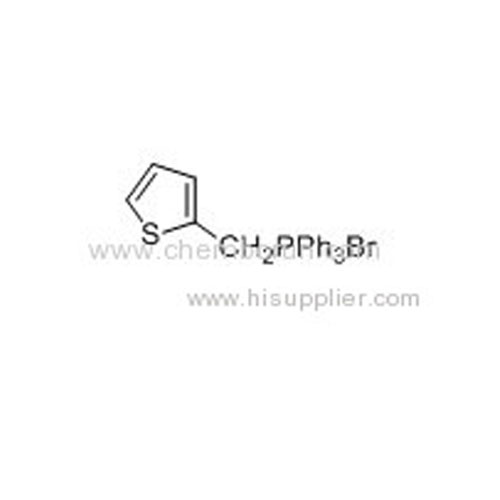
2-Thienyltriphenylphosphonium bromide -
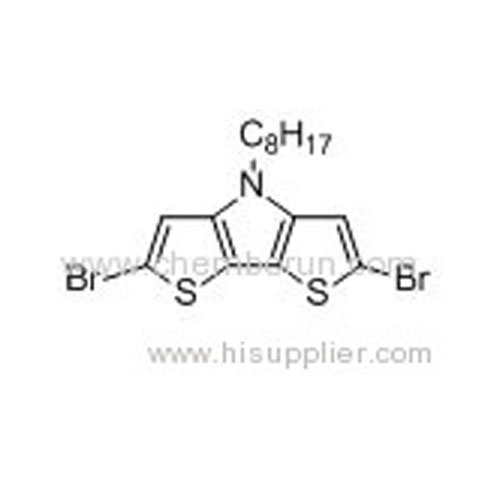
2,6-Dibromo-N-octyl-dithieno[3,2-b:2,3-d]pyrrole -
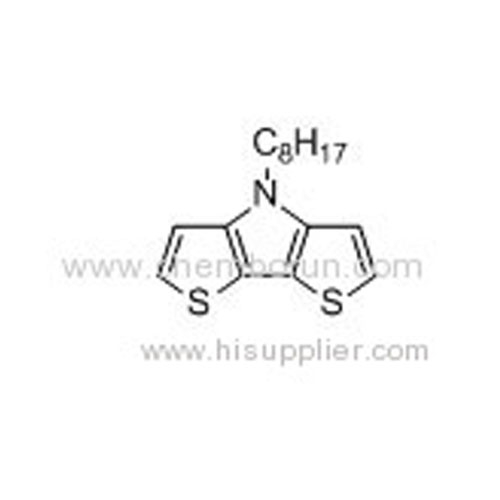
N-octyl-dithieno[3,2-b:2,3-d]pyrrole -
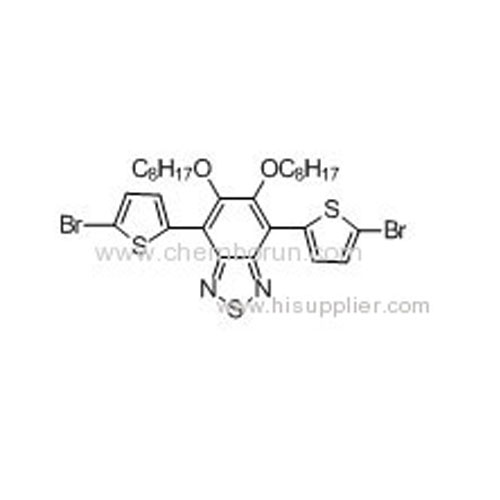
4,7-Bis(5-bromothiophen-2-yl)5,6-bis(octyloxy)benzo-2,1,3-thiadiazole -
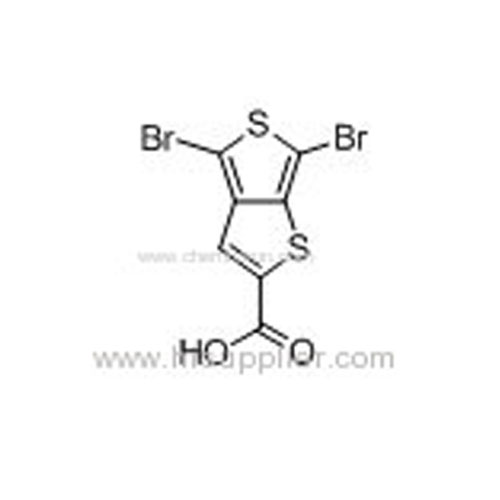
4,6-Dibromo-thieno[3,4-b]thiophene-2-carboxylic acid -
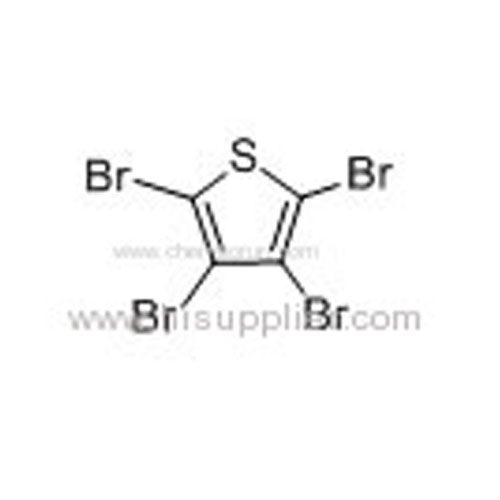
Tetrabromothiophene -
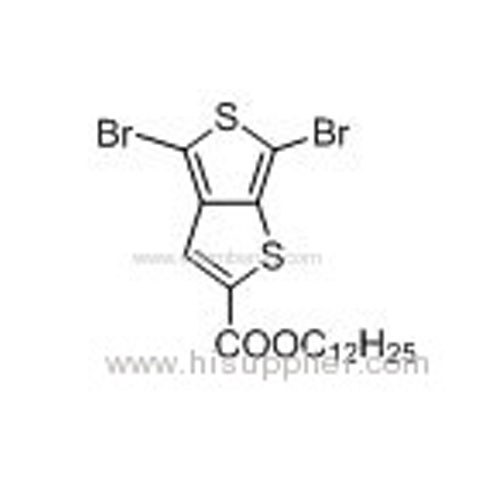
Dodecyl 4,6-dibromothieno[3,4-b]thiophene-2-carboxylate -
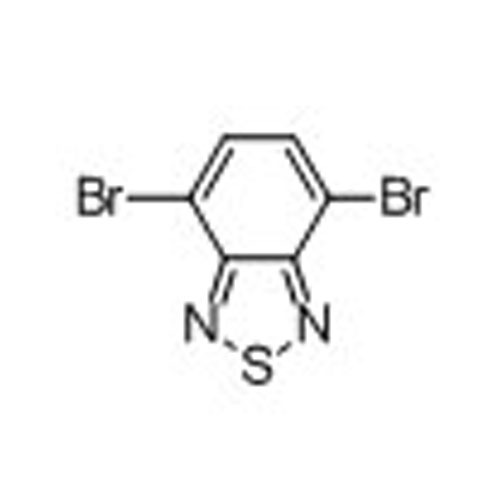
4,7-Dibromobenzo[c][1,2,5]thiadiazole -
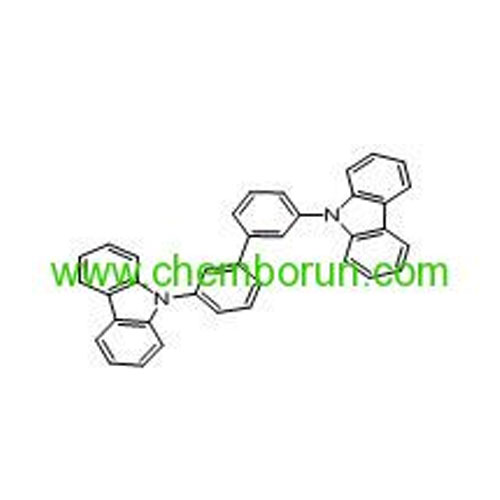
9,9'-biphenyl-3,3'-diylbis-9H-carbazole -
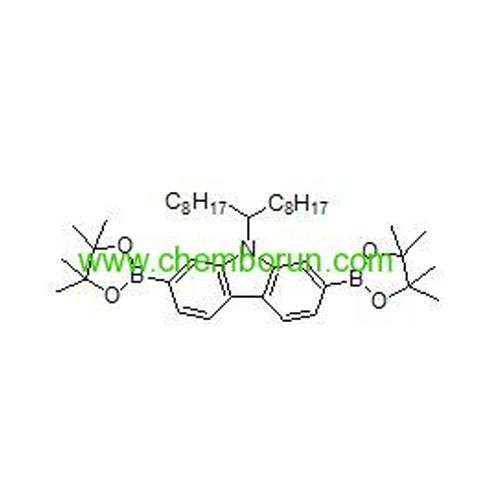
9-(heptadecan-9-yl)-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole -
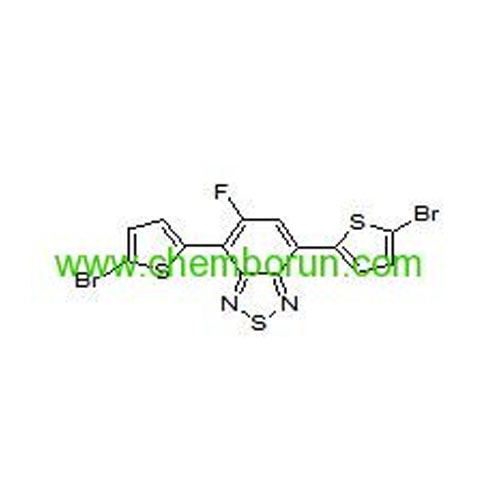
4,7-bis(5-bromothiophen-2-yl)-5-fluorobenzo[c][1,2,5]thiadiazole -
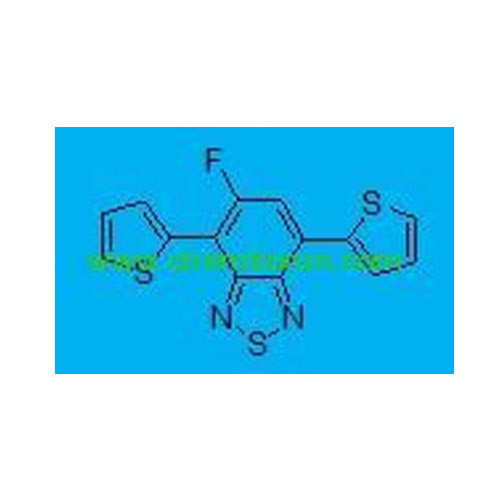
5-fluoro-4,7-di(thiophen-2-yl)benzo[c][1,2,5]thiadiazole -
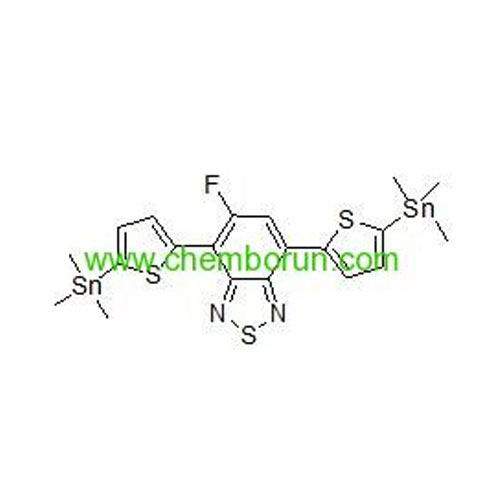
5-fluoro-4,7-bis(5-(trimethylstannyl)thiophen-2-yl)benzo[c][1,2,5]thiadiazole -
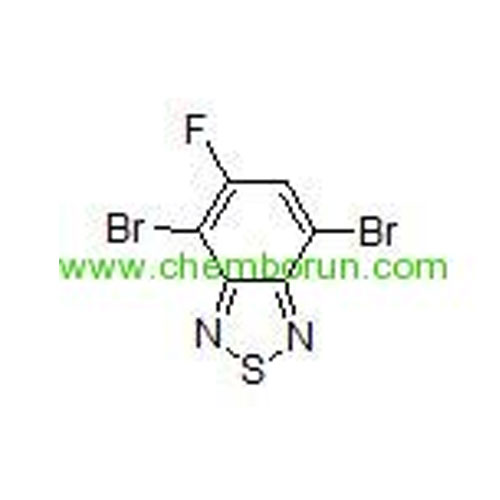
4,7-dibromo-5-fluorobenzo[c][1,2,5]thiadiazole -
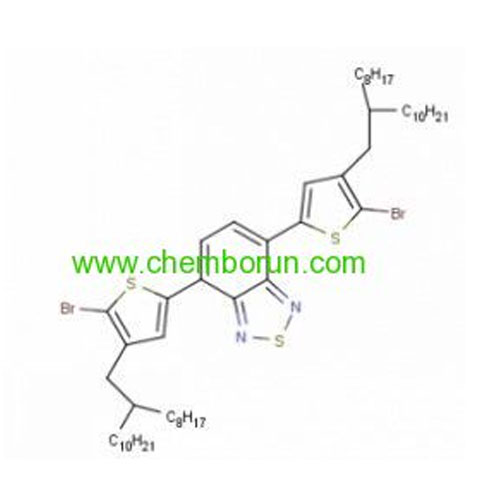
4,7-Bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole -
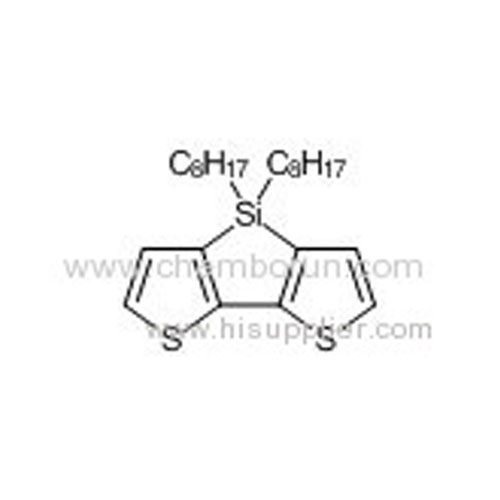
4,4'-Bis(octyl)-dithieno[3,2-b:2',3'-d]silole -
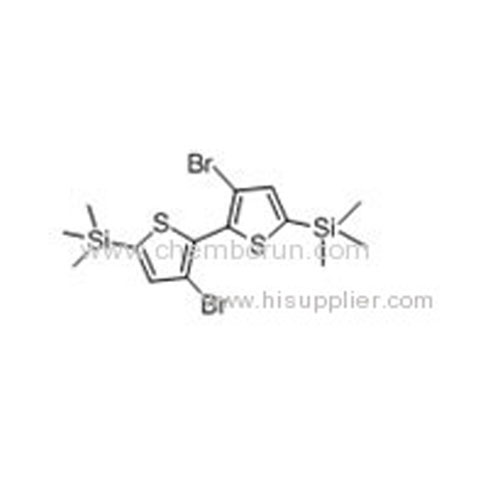
3,3'-Dibromo-5,5'-bis-trimethylsilyl-2,2'-bithiophene -
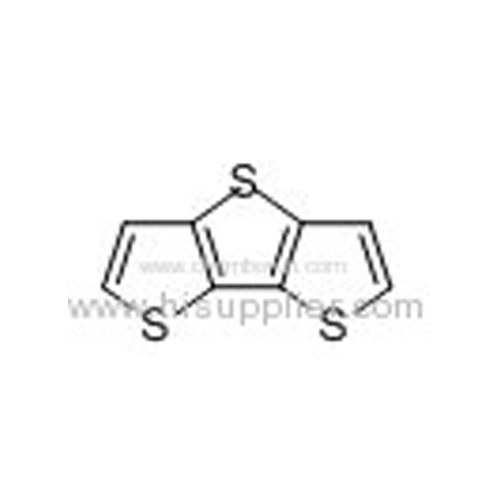
Dithieno[3,2-b:2',3'-d]thiophene -
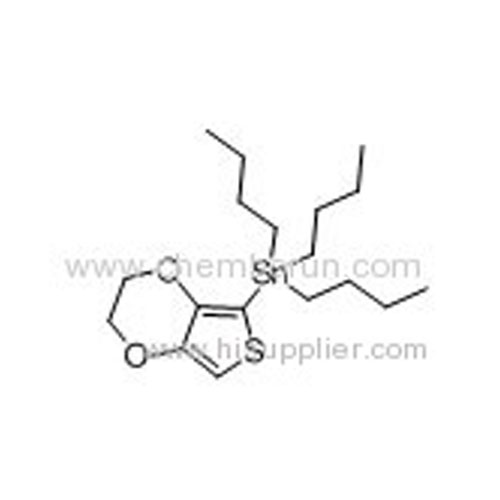
Tributyl(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)stannane -
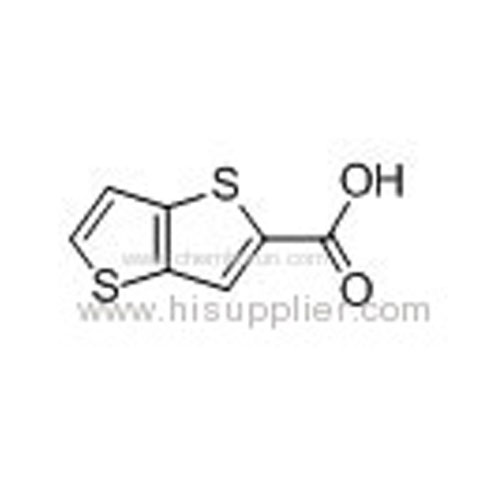
Thieno[3,2-b]thiophene-2-carboxylic acid -
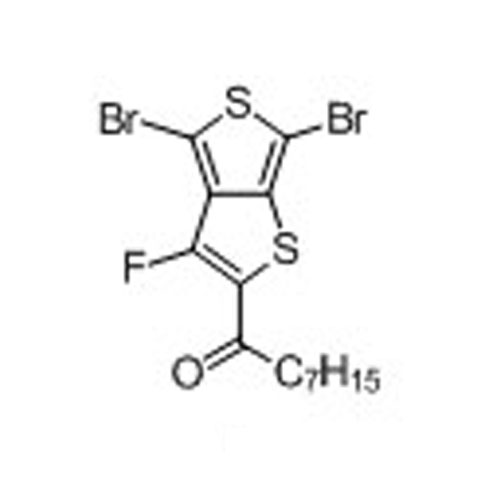
1-(4,6-Dibromo-3-fluorothieno[3,4-b]thiophen-2-yl)octan-1-one -
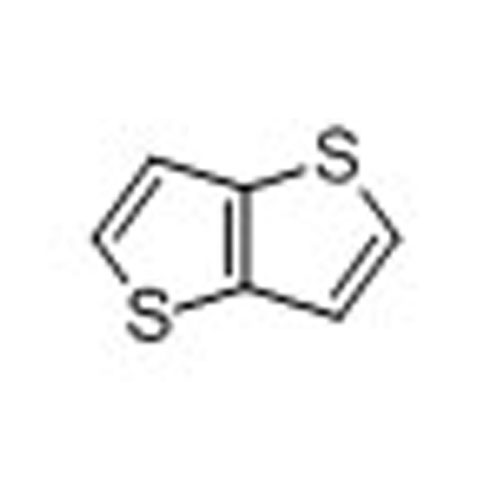
Thieno[3,2-b]thiophene -
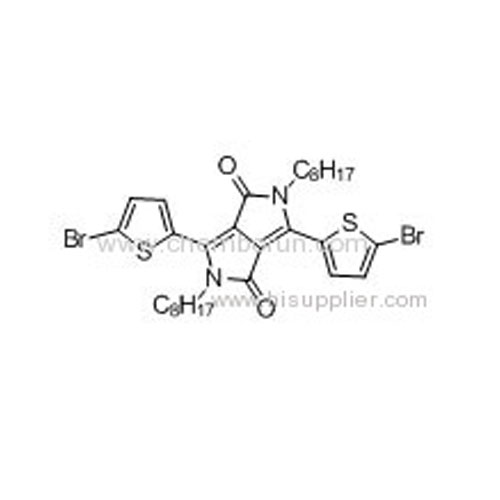
3,6-Bis(5-bromothiophen-2-yl)-2,5-dioctylpyrrolo[3,4-c]pyrrole-1,4-dione -
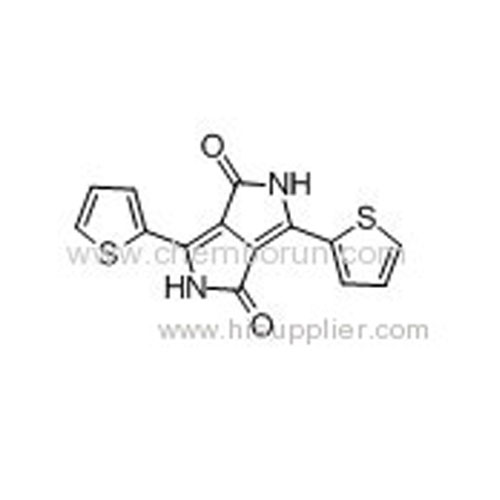
3,6-Di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4-dione -
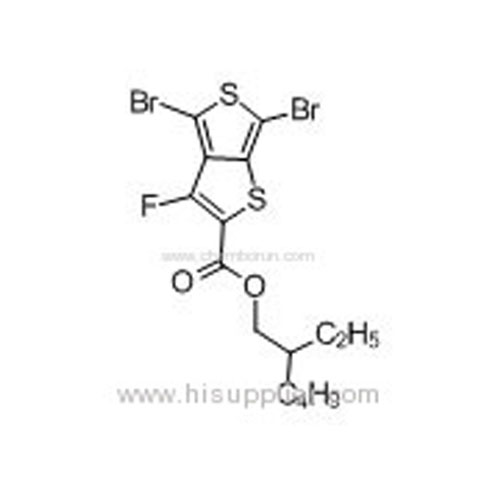
2-Ethylhexyl-4,6-dibromo-3-fluorothieno[3,4-b]thiophene-2-carboxylate -
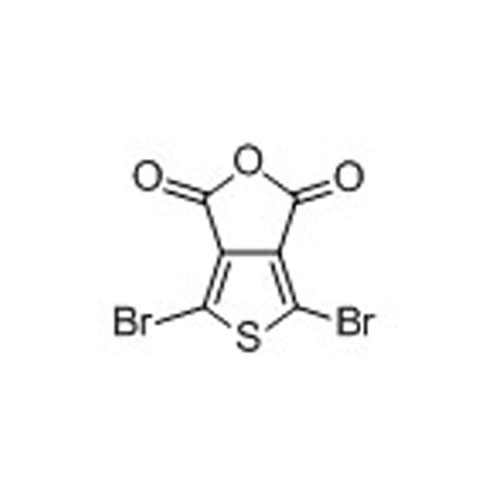
4,6-Dibromothieno[3,4-c]furan-1,3-dione -
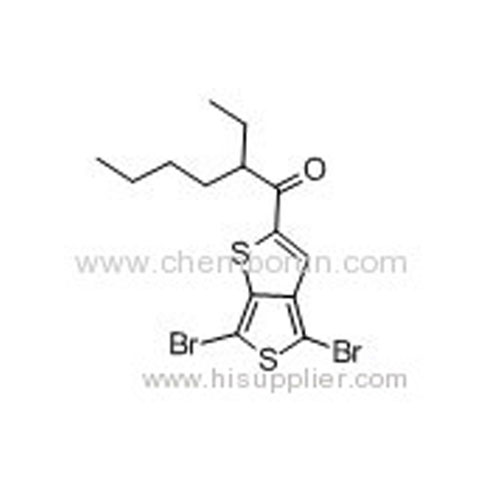
1-(4,6-Dibromothieno[3,4-b]thiophen-2-yl)-2-ethylhexan-1-one -
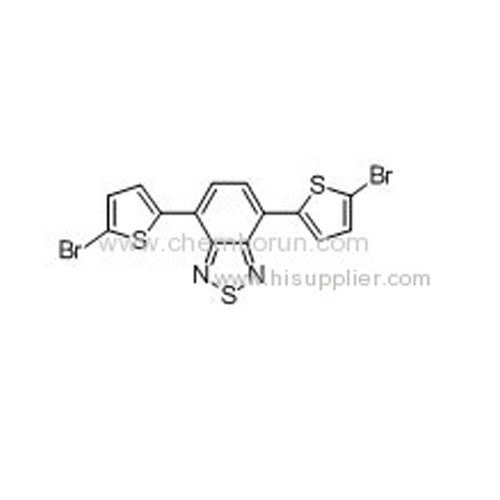
4,7-Bis(2-bromo-5-thienyl)-2,1,3-benzothiadiazole -
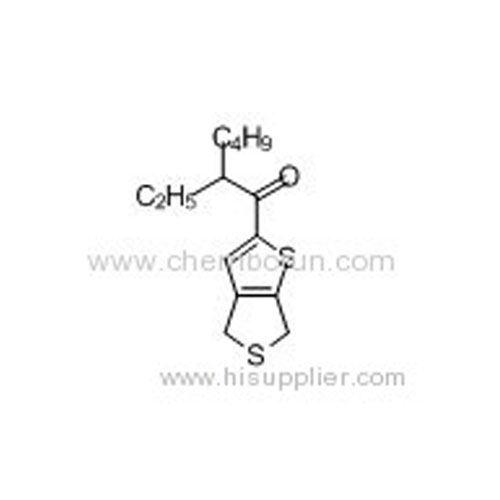
1-(4,6-Dihydrothieno[3,4-b]thiophen-2-yl)-2-ethylhexan-1-one -
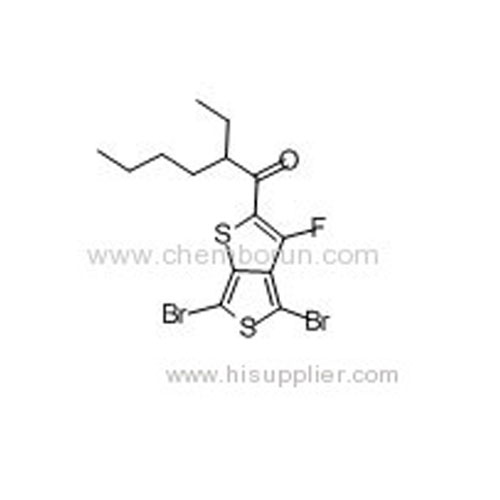
1-(4,6-Dibromo-3-fluorothieno[3,4-b]thiophen-2-yl)-2-ethylhexan-1-one -
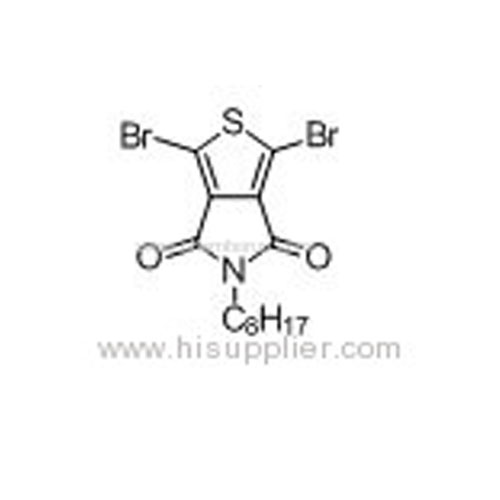
1,3-Dibromo-5-octyl-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione -
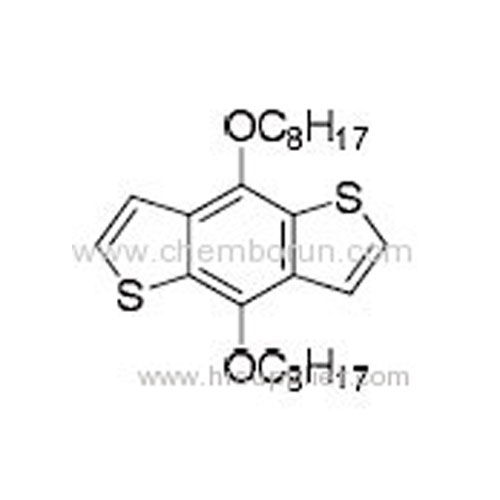
4,8-Dioctyloxybenzo[1,2-b:3,4-b']dithiophene -
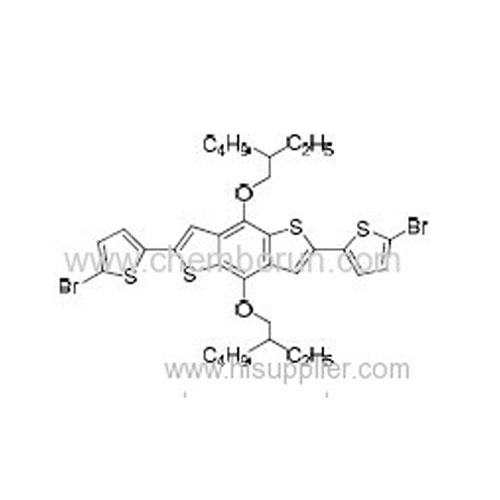
2,6-Bis(5-bromothiophen-2-yl)-4,8-bis((2-ethylhexyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
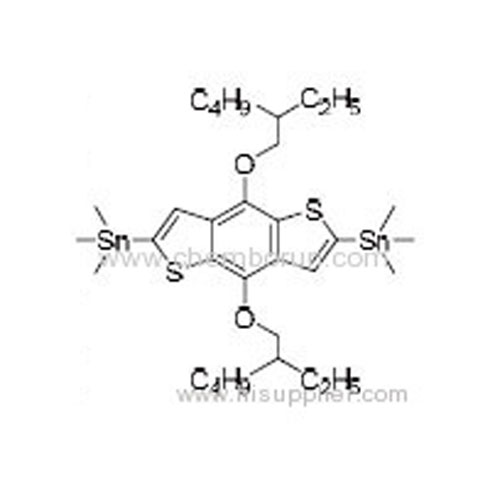
2,6-Bis(trimethyltin)-4,8-bis(2-ethylhexyloxy)benzo[1,2-b:4,5-b']dithiophene -
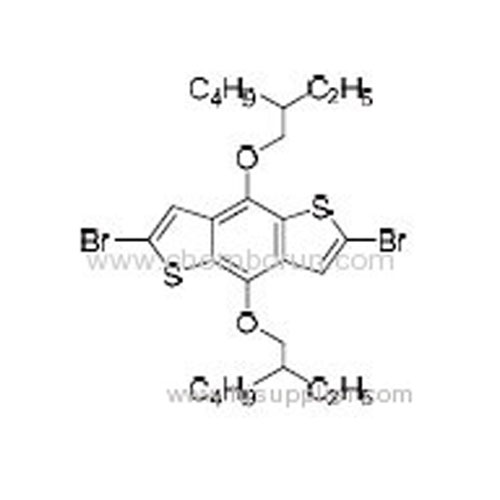
2,6-Dibromo-4,8-bis(2-ethylhexyloxy)benzo[1,2-b:4,5-b']dithiophene -
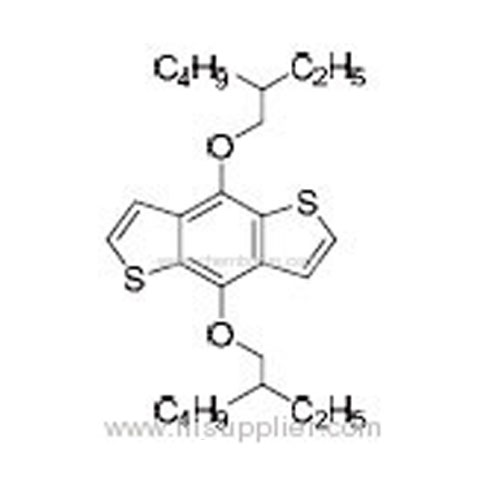
4,8-Bis(2-ethylhexyloxy)benzo[1,2-b:4,5-b']dithiophene -
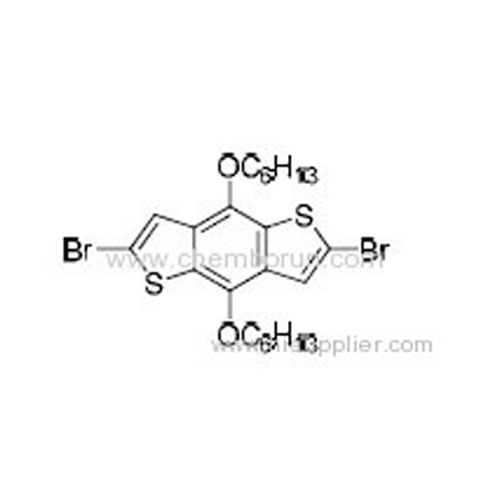
2,6-Dibromo-4,8-bis(hexyloxy)benzo[1,2-b:4,5-b']dithiophene -
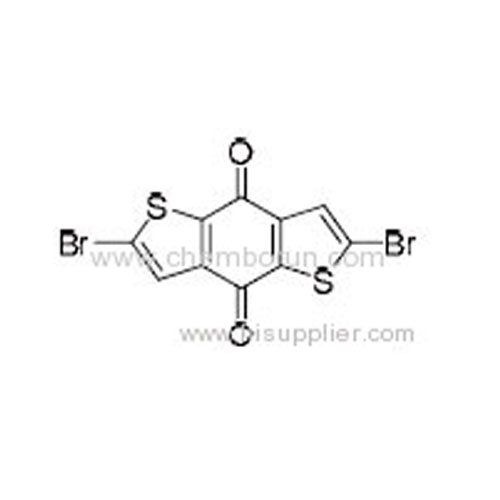
2,6-Dibromobenzo[1,2-b:4,5-b']dithiophene-4,8-dione -
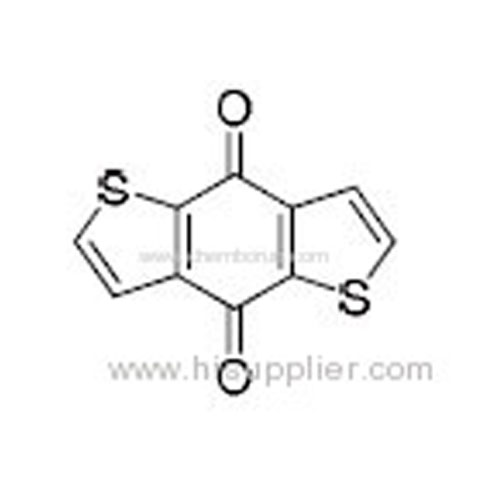
4,8-Dihydrobenzo[1,2-b:4,5-b']dithiophene-4,8-dione -
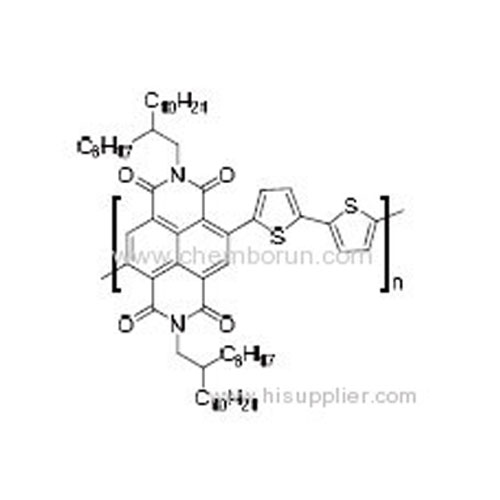
Poly(2,7-bis(2-octyldodecyl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraone-4,9-diyl)([2,2']bithiophenyl-5,5'-diyl -
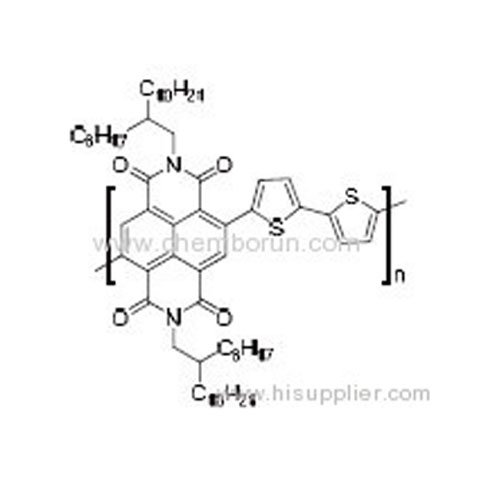
Poly(2,7-bis(2-octyldodecyl)benzo[lmn][3,8]phenanthroline-1,3,6,8 (2H,7H)-tetraone-4,9-diyl)([2,2']bithiophenyl-5,5'-diy -
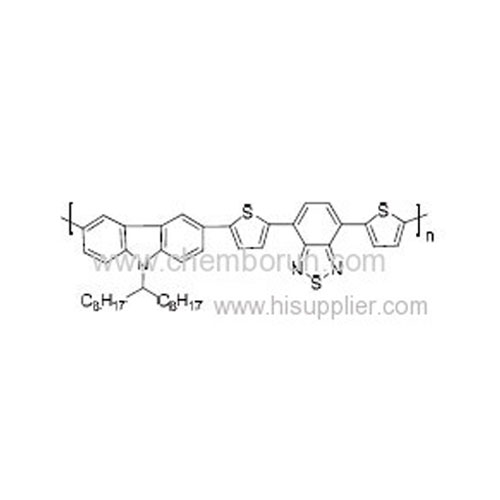
Poly[[9-(1-octylnonyl)-9H-carbazole-2,7-diyl]-2,5-thiophenediyl-2,1,3-benzo thiadiazole-4,7-diyl-2,5-thiophenediyl] -
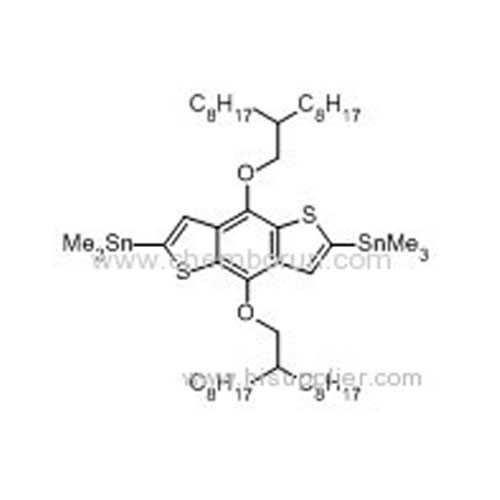
(4,8-Bis((2-octyldecyl)oxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
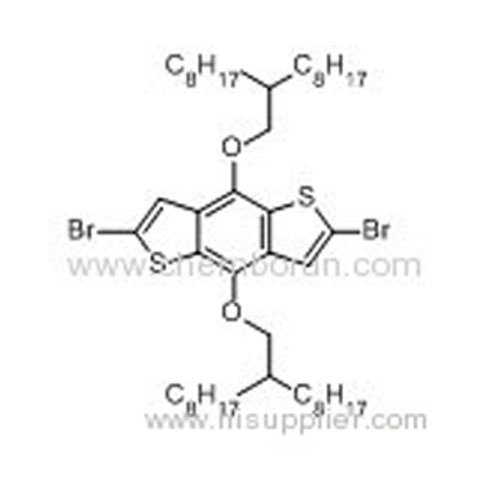
2,6-Dibromo-4,8-bis((2-octyldecyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
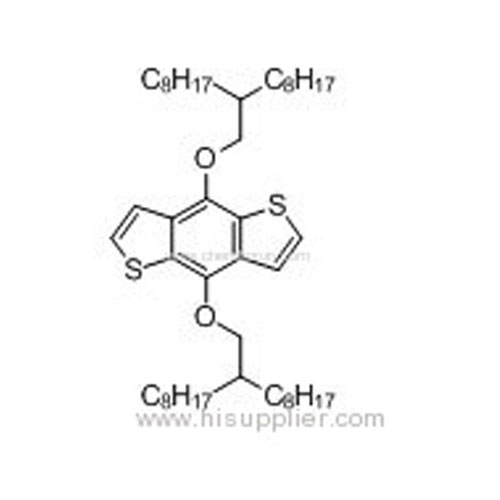
4,8-Bis((2-octyldecyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
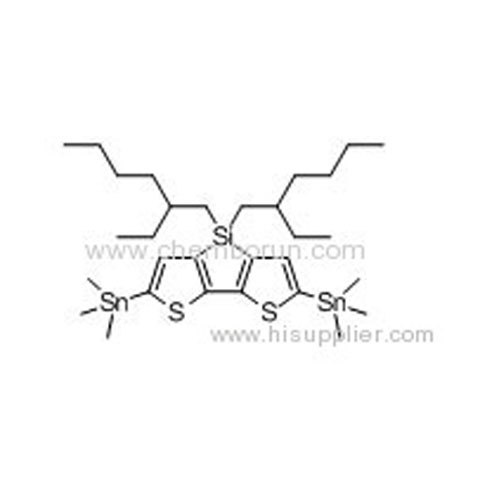
4,4'-Bis(2-ethyl-hexyl)-5,5'-bis(trimethyltin)-dithieno[3,2-b:2,3-d]silole -
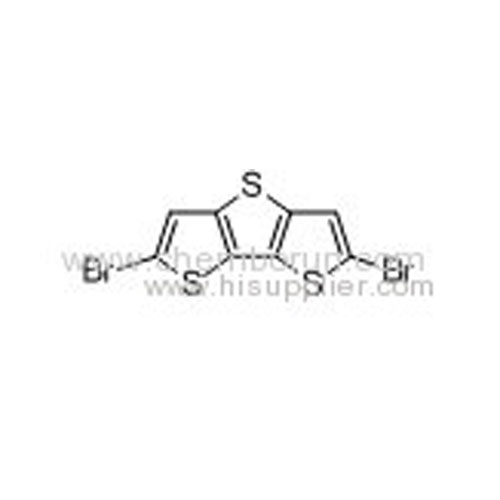
2,6-Dibromo-dithieno[3,2-b:2,'3'-d]thiophene -
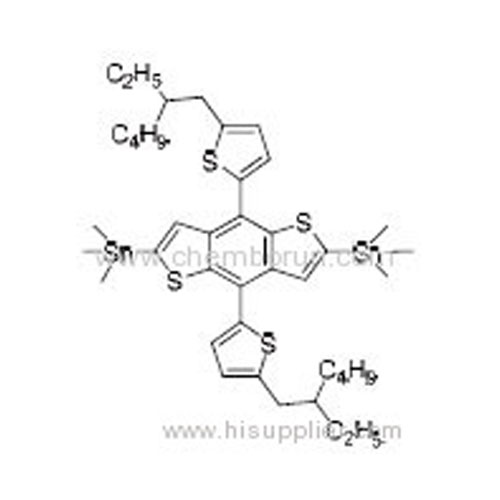
2,6-Bis(trimethyltin)-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
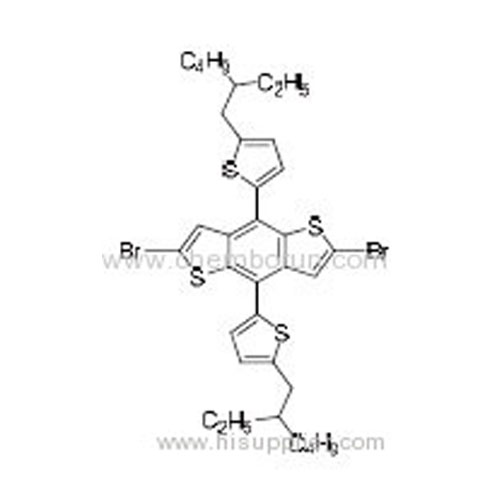
2,6-Dibromo-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
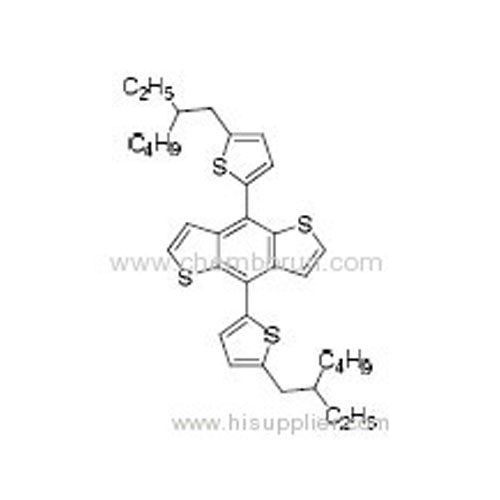
4,8-Bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
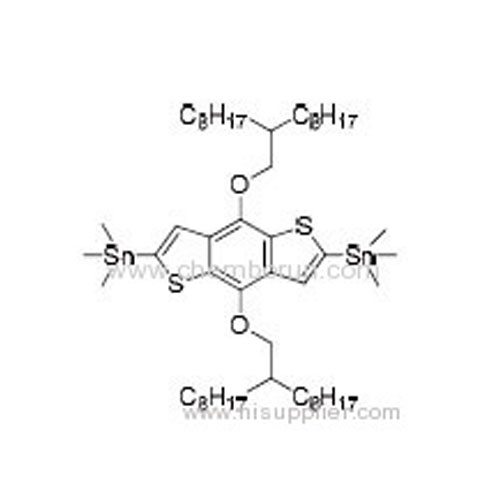
(4,8-Bis((2-octyldecyl)oxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diy)bis (trimethylstannane) -
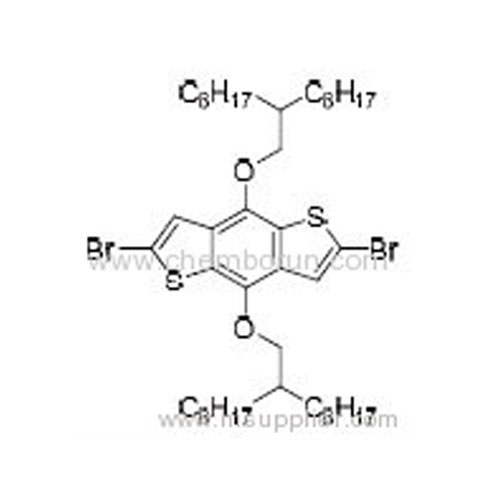
2,6-Dibromo-4,8-bis((2-octyldecyl)oxy)benzo[1,2-b:4,5-b'] dithiophene -
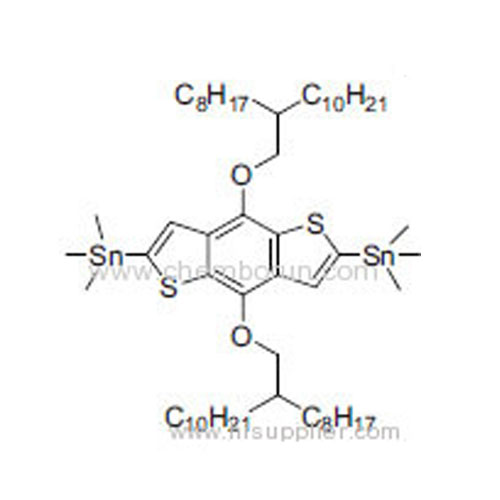
(4,8-Bis((2-octyldodecyl)oxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
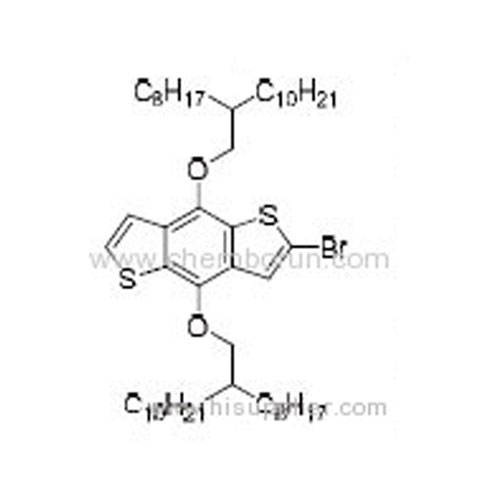
2-Bromo-4,8-bis((2-ctyldodecyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
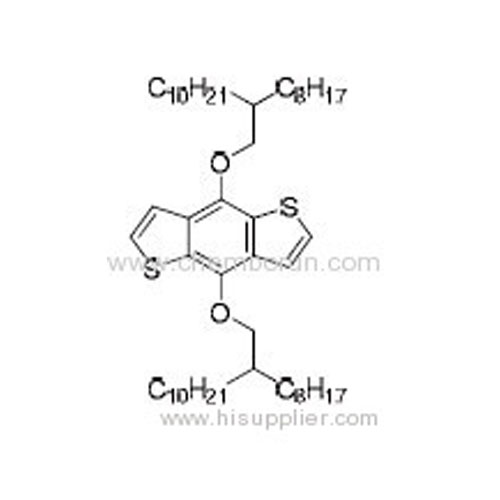
4,8-Bis((2-octyldodecyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
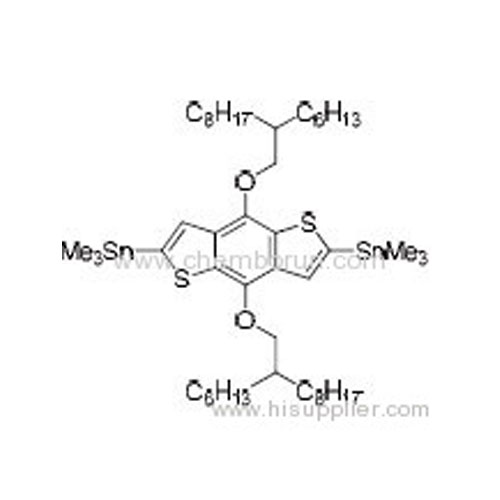
(4,8-Bis((2-hexyldecyl)oxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane -
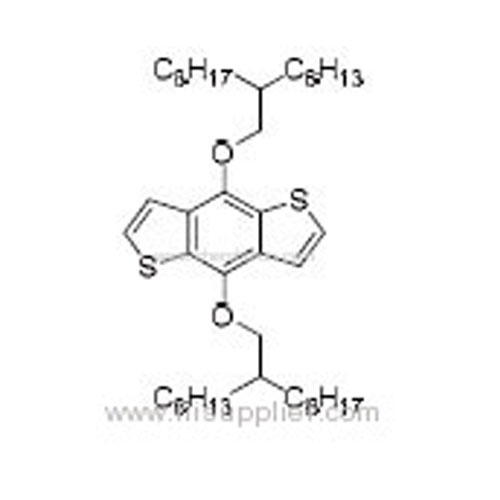
4,8-Bis((2-hexyldecyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
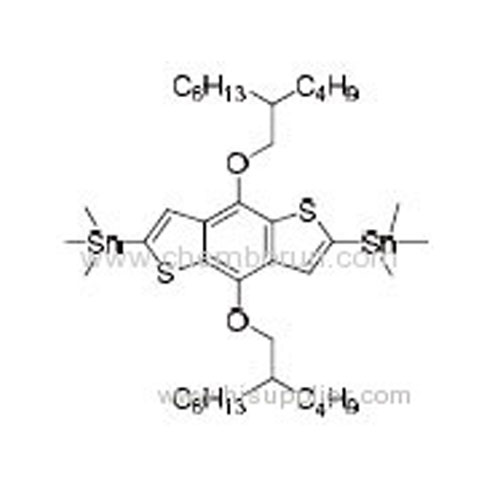
(4,8-Bis((2-butyloctyl)oxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
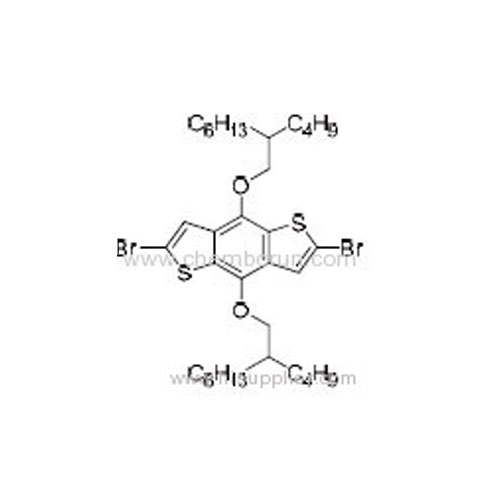
2,6-Dibromo-4,8-bis((2-butyloctyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
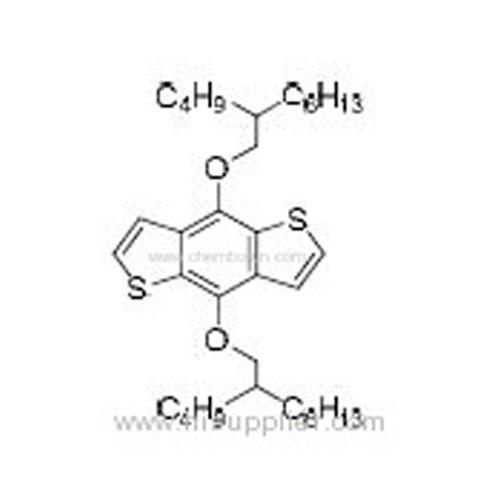
4,8-Bis((2-butyloctyl)oxy)benzo[1,2-b:4,5-b']dithiophene -
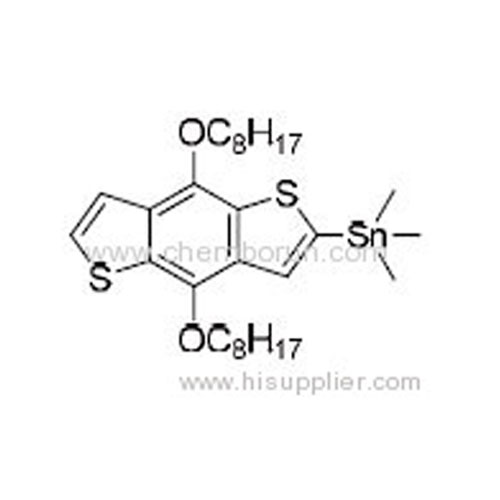
(4,8-Bis(octyloxy)benzo[1,2-b:4,5-b']dithiophen-2-yl)trimethylstannane -
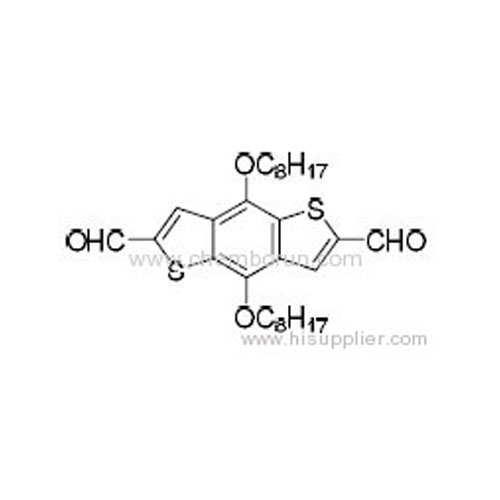
2,6-Dicarbaldehyde-4,8-dioctyloxybenzo[1,2-b:3,4-b']dithiophene -
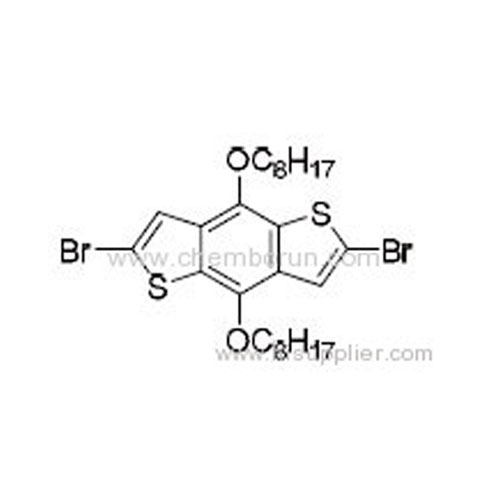
2,6-Dibromo-4,8-bis(octyloxy)benzo[1,2-b:4,5-b']dithiophene -
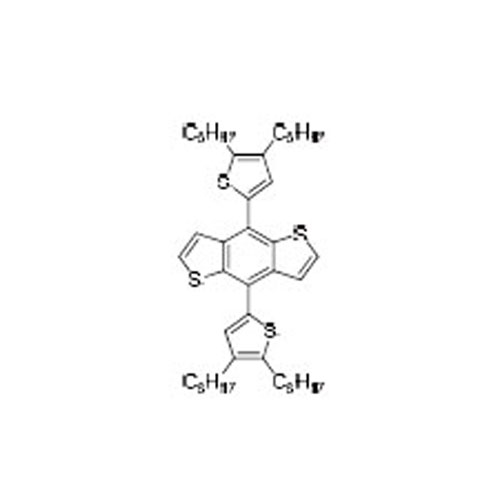
4,8-Bs(4,5-dioctylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
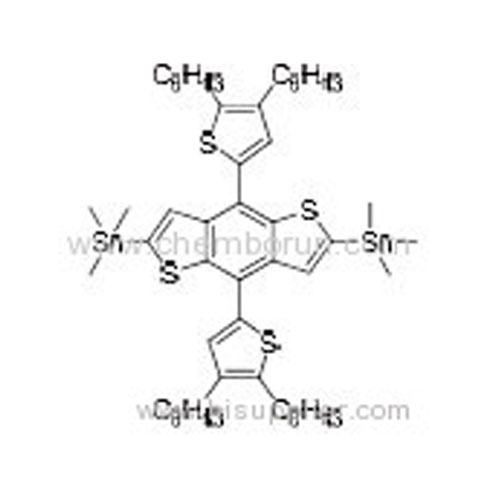
(4,8-Bis(4,5-dihexylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
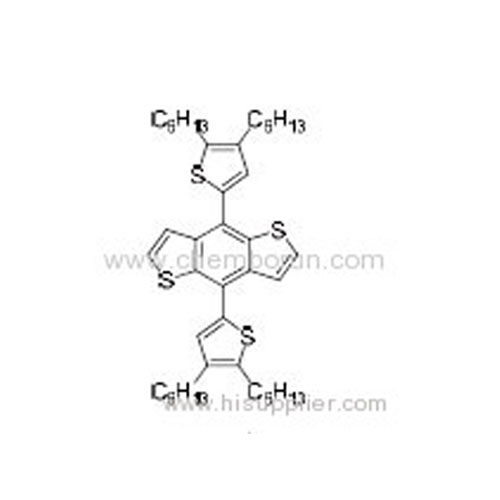
4,8-Bis(4,5-dihexylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
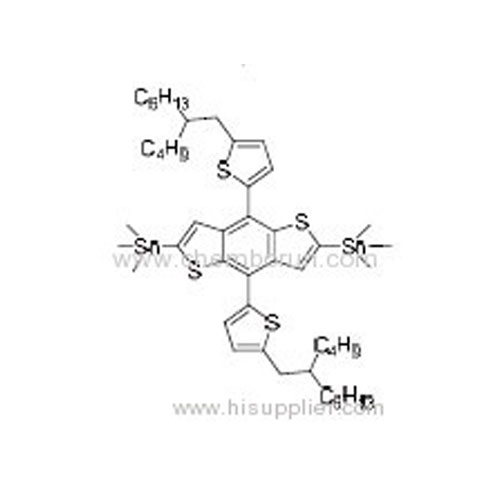
(4,8-Bis(5-(2-butyloctyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
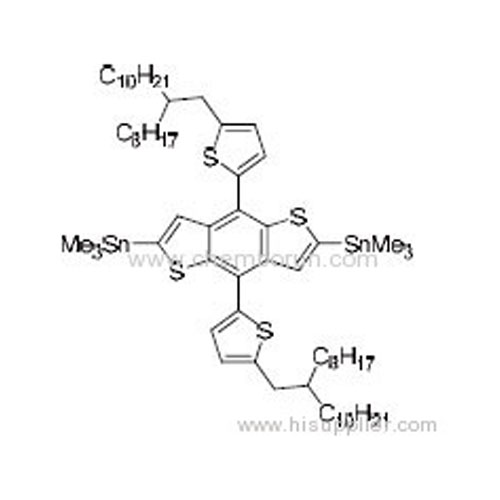
(4,8-Bis(2-(5-(2-octyldodecy))thiophene)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
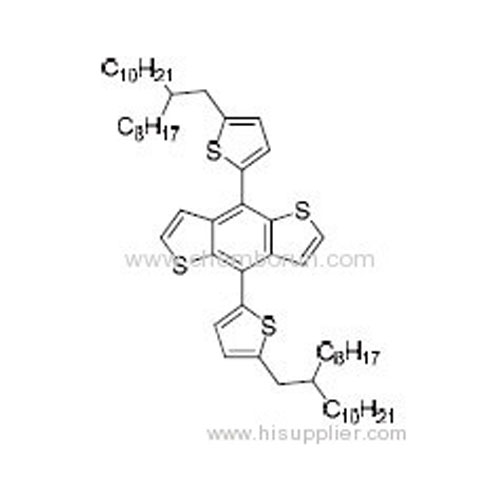
4,8-Bis(5-(2-octyldodecyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
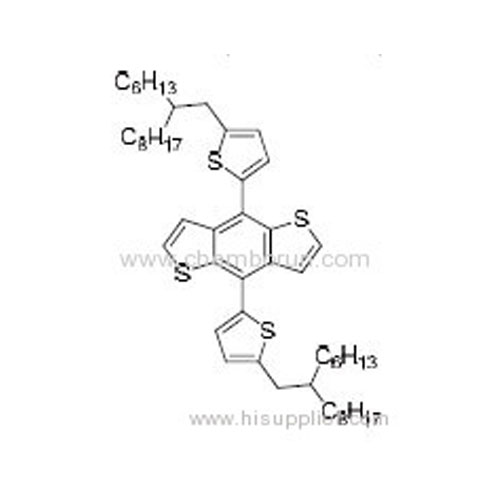
4,8-Bis(5-(2-hexyldecyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
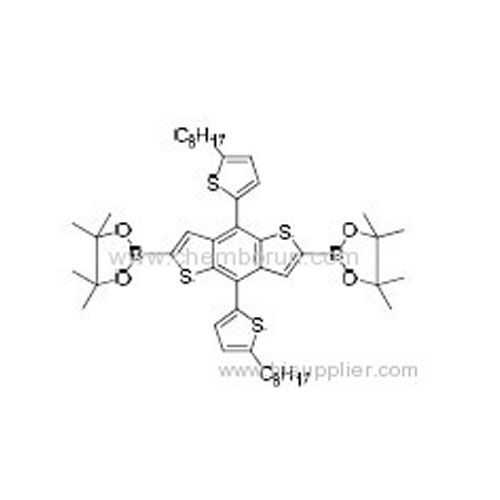
2,2'-(4,8-Bis(5-octylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) -
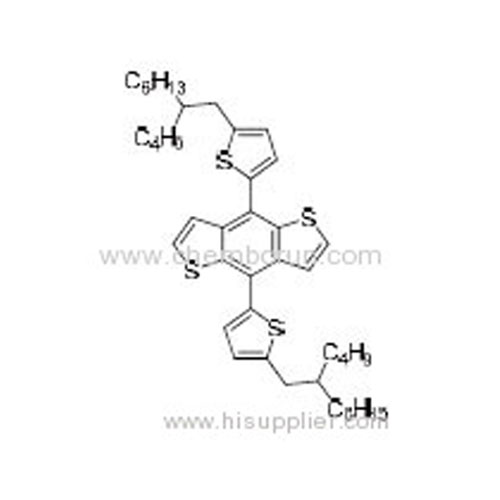
(4,8-Bis(5-octylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
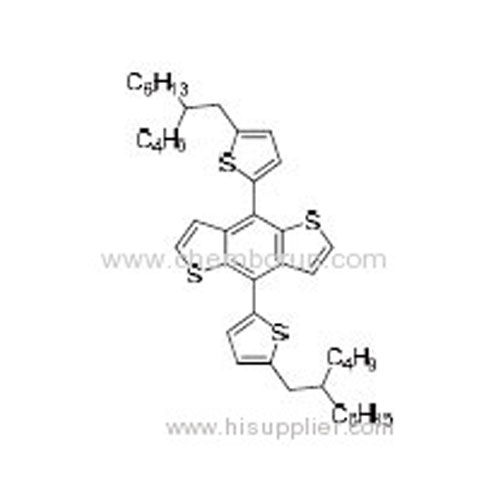
4,8-Bis(5-(2-butyloctyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
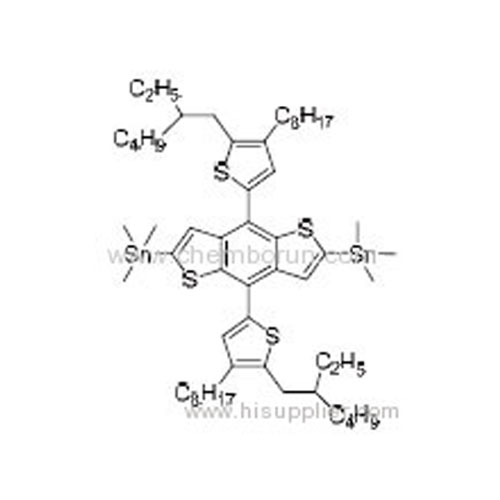
(4,8-Bis(5-(2-ethylhexyl)-4-octylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
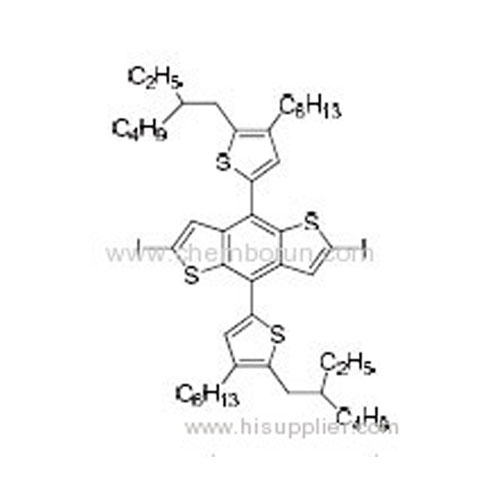
4,8-Bis(5-(2-ethylhexyl)-4-hexylthiophen-2-yl)-2,6-diiodobenzo[1,2-b:4,5-b']dithiophene -
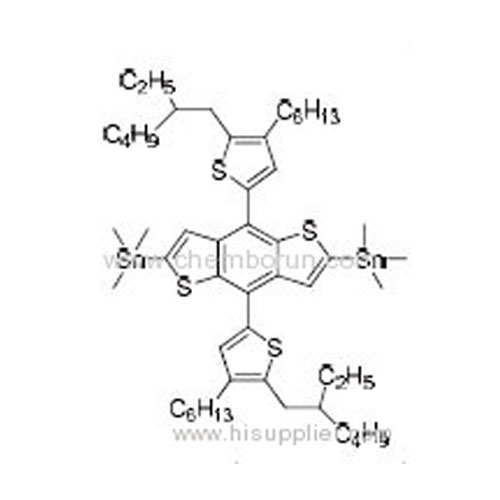
(4,8-Bis(5-(2-ethylhexyl)-4-hexylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
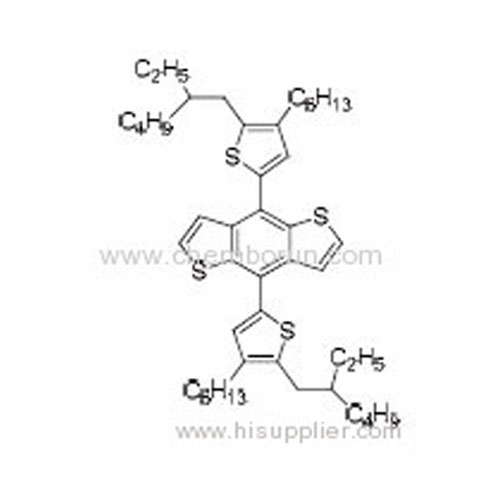
4,8-Bis(5-(2-ethylhexyl)-4-hexylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
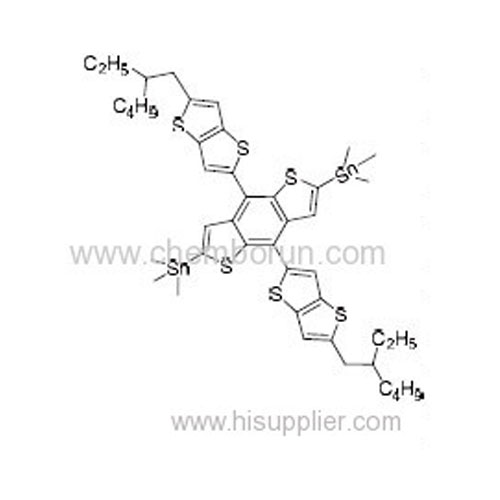
4,8-Bis(5-(2-ethylhexyl)thieno[3,2-b]thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
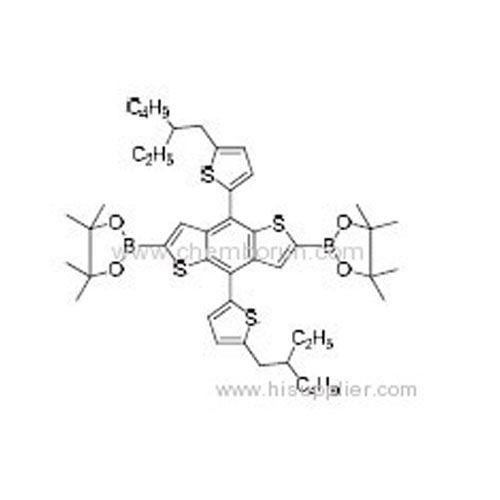
2,2'-(4,8-Bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxab -
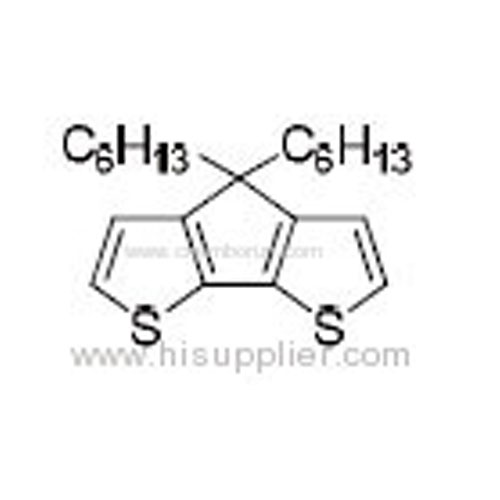
2,6-Dibromo-4,4-dihexyl-4H-cyclopenta[2,1-b:3,4-b']dithiophene -
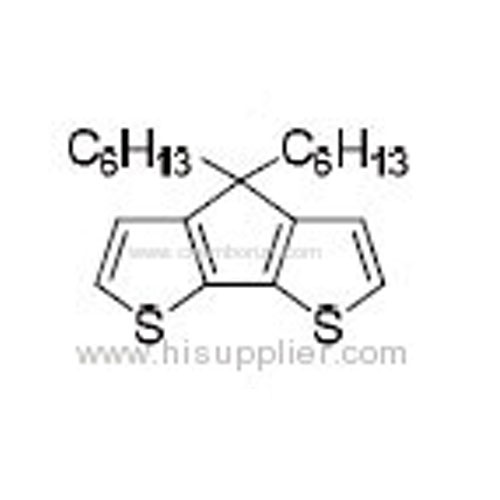
4,4-Dihexyl-4H-cyclopenta[2,1-b:3,4-b']dithiophene -
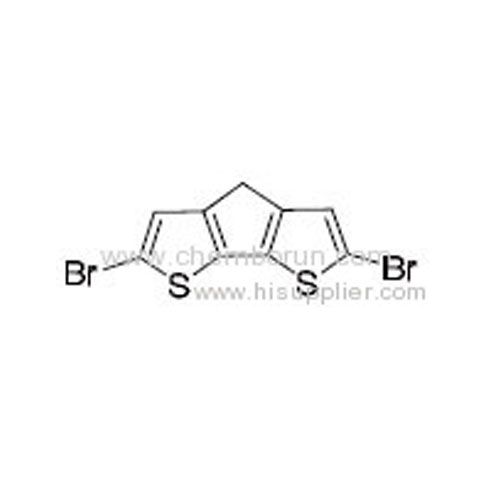
2,6-Dibromo-3,4-dithia-7H-cyclopenta[a]pentalene -
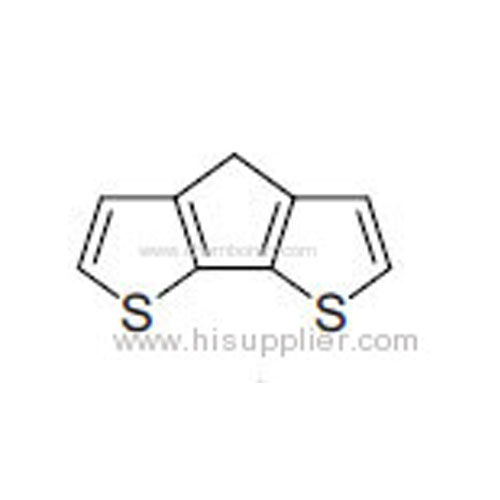
4H-Cyclopenta[2,1-b:3,4-b']dithiophene -
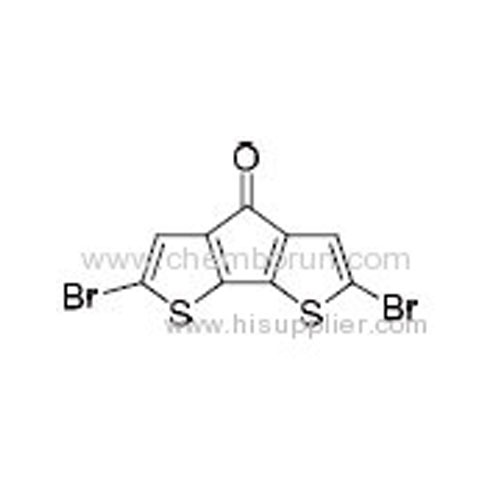
2,6-Dibromo-4H-cyclopenta[1,2-b:5,4-b']dithiophen-4-one -
4H-Cyclopenta[2,1-b:3,4-b']dithiophene-4-one -
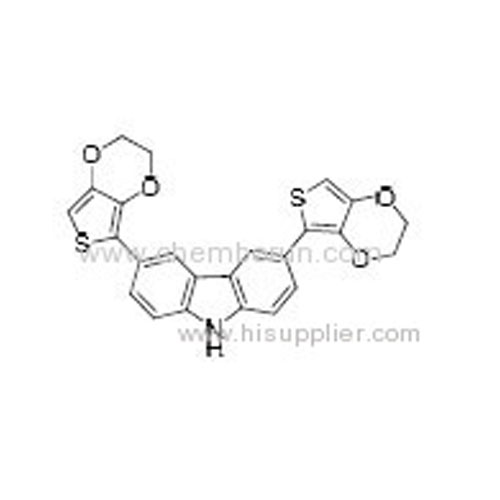
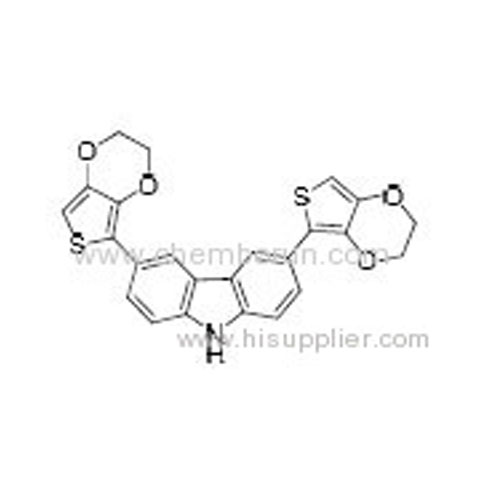
3,6-Bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)-9H-carbazole -
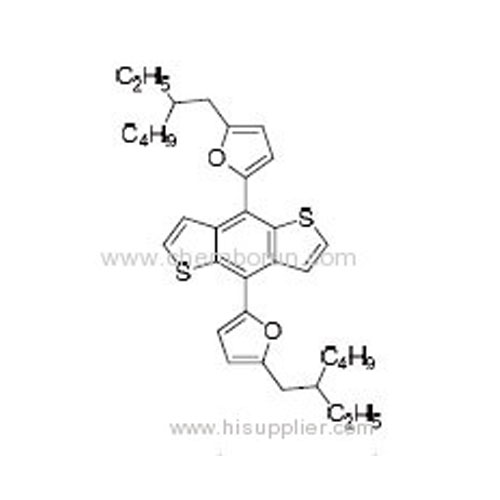
4,8-Bis(5-(2-ethylhexyl)furan-2-yl)benzo[1,2-b:4,5-b']dithiophene -
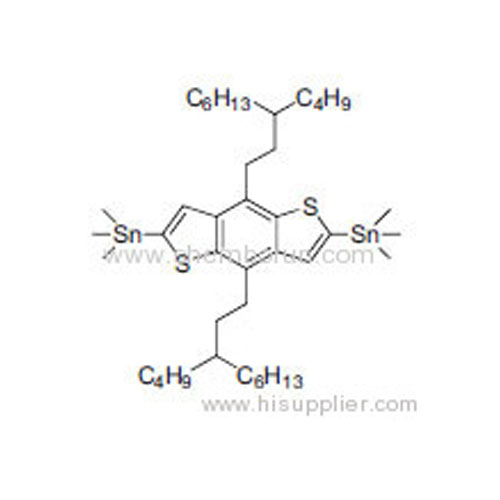
(4,8-Bis(3-butylnonyl)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
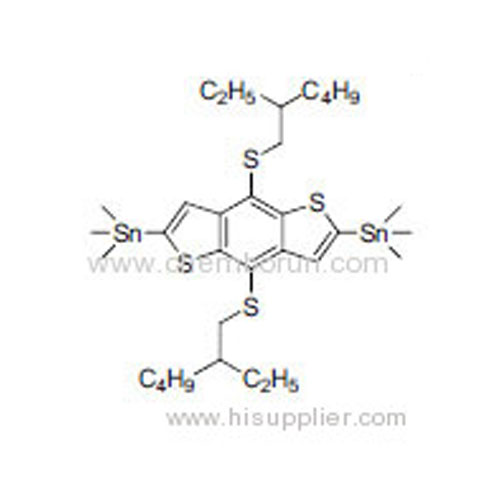
(4,8-Bis(3-ethylheptyl))benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
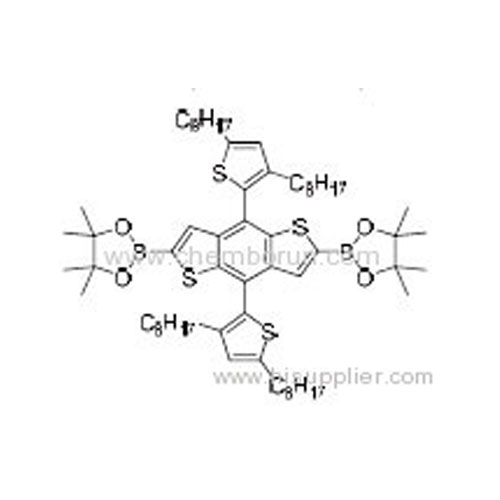
2,6-Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-(4,8-bis(2-(3,5-dioctyl)thiophene)benzo[1,2-b:4,5-b']dithiophene -
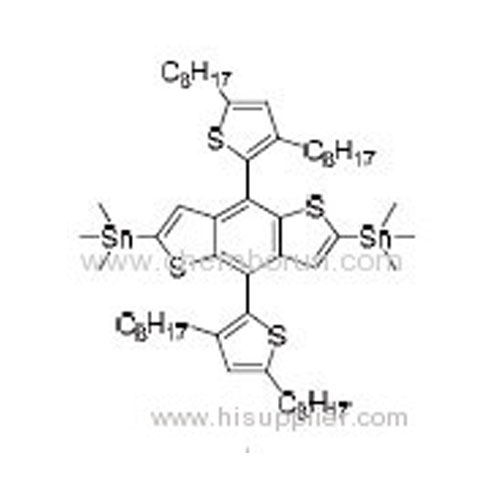
(4,8-Bis(2-(3,5-dioctyl)thiophene)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
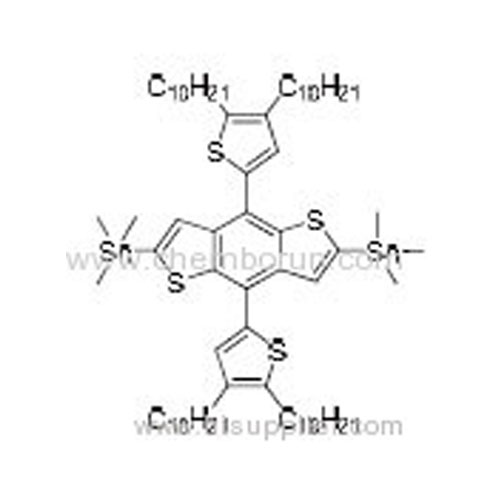
(4,8-Bis(2-(3,5-didecyl)thiophene)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
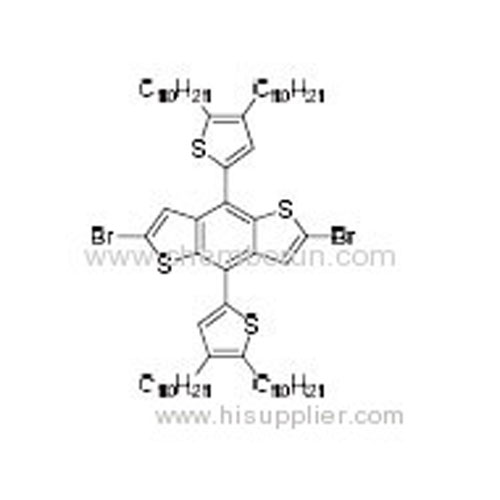
2,6-Dibromo-4,8-bis(4,5-didecylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
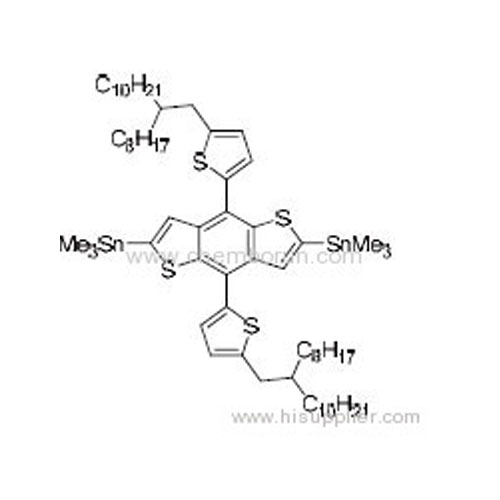
(4,8-Bis(2-(5-(2-octyldodecyl))thiophene)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethylstannane) -
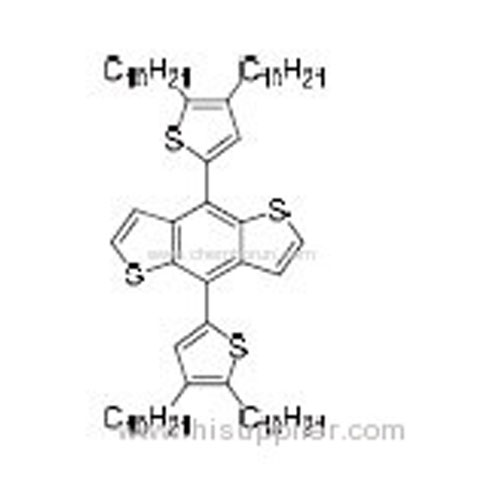
4,8-Bis(4,5-didecylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene -
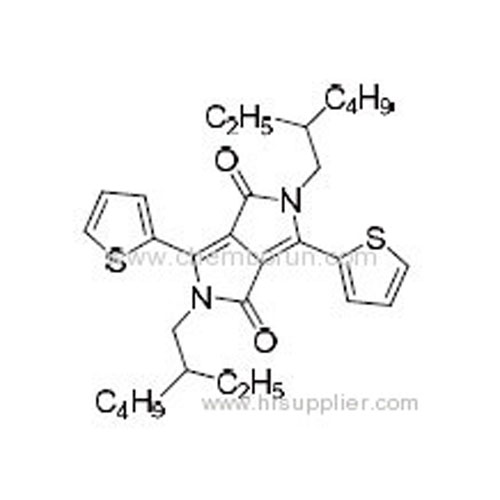
2,5-Bis(2-ethylhexyl)-3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
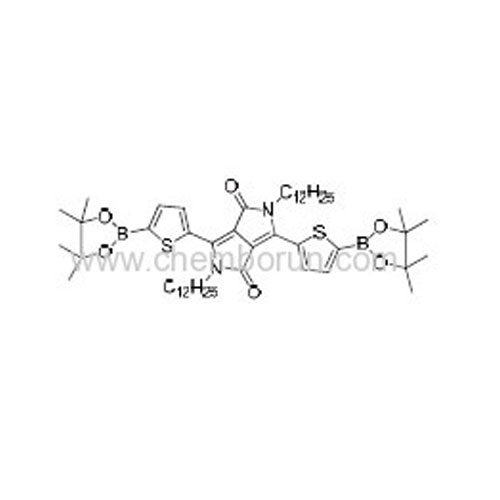
2,5-Didodecyl-3,6-bis(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dion -
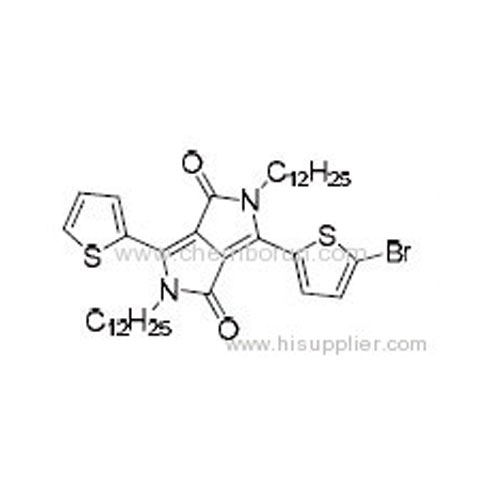
3-(5-Bromothiophen-2-yl)-2,5-didodecyl-6-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
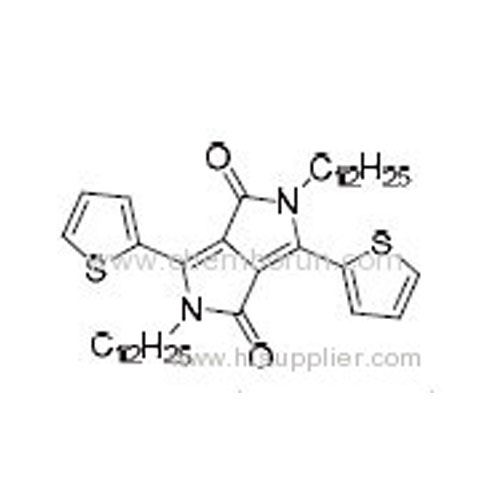
2,5-Didodecyl-3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
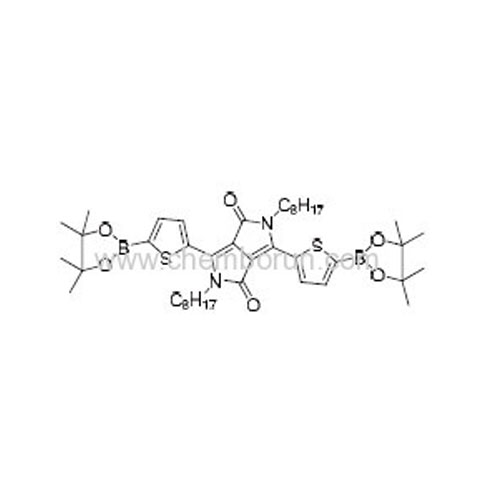
2,5-Dioctyl-3,6-bis(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
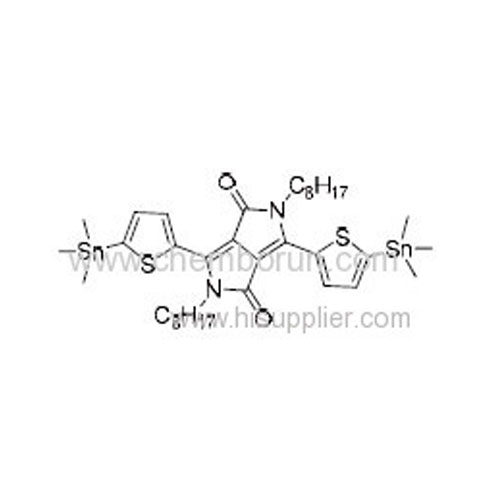
2,5-Dioctyl-3,6-bis(5-(trimethylstannyl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
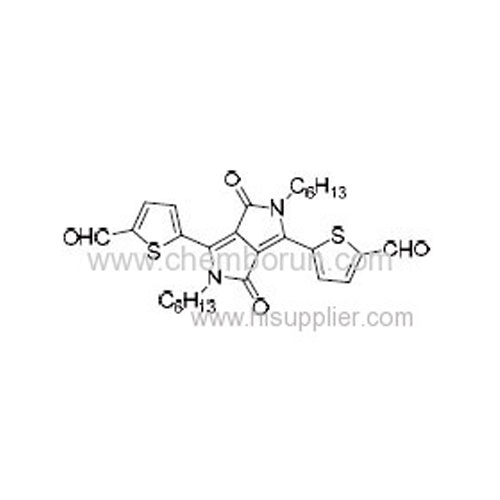
5,5'-(2,5-Dihexyl-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrole-1,4-diyl)bis(thiophene-2-carbaldehyde) -
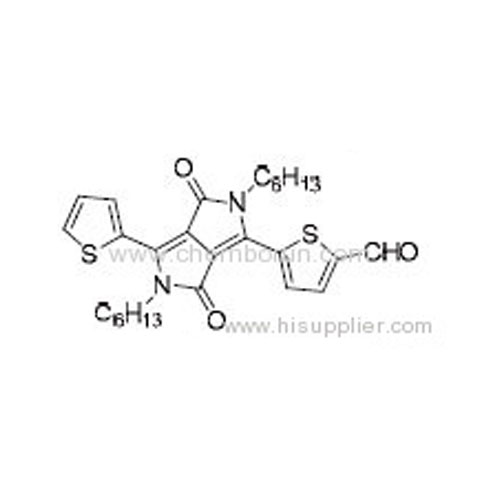
5-(2,5-Dihexyl-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophene-2-carbaldehyde -
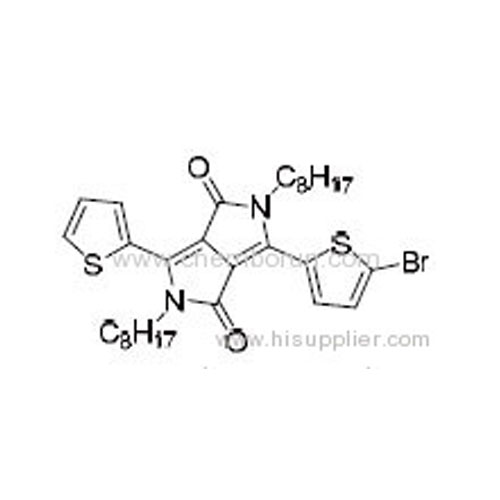
3-(5-Bromothiophen-2-yl)-2,5-dioctyl-6-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
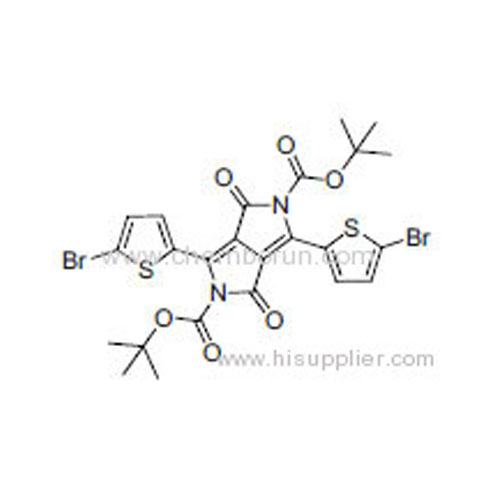
Di-tert-butyl 3,6-bis(5-bromothiophen-2-yl)-1,4-dioxopyrrolo[3,4-c]pyrrole-2,5(1H,4H)-dicarboxylate -
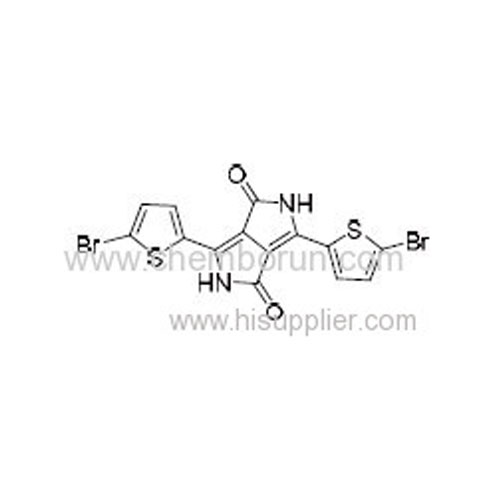
3,6-Bis(5-bromothiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
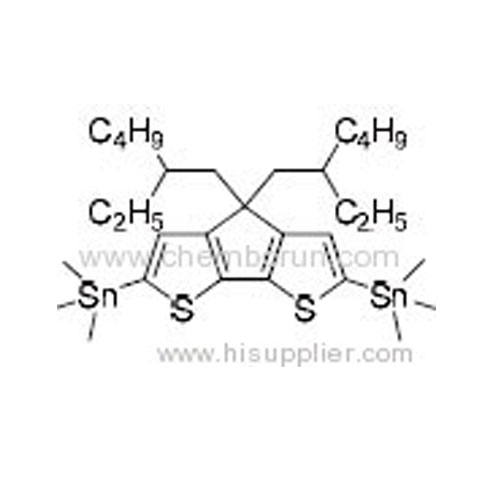
2,6-Bis(trimethyltin)-4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene -
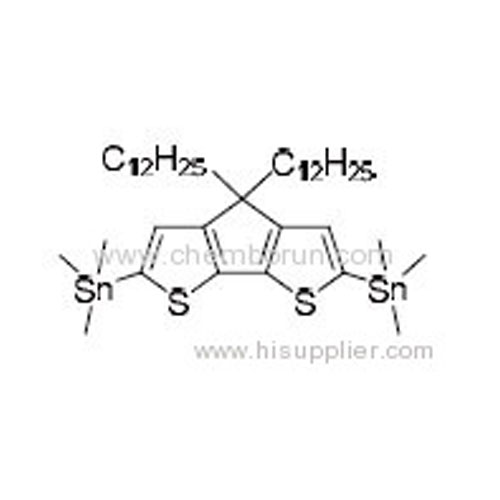
2,6-Bis(trimethyltin)-4,4-bis(2-dodecylbenzo)-4H-cyclopenta[2,1-b:3,4-b']dithiophene -
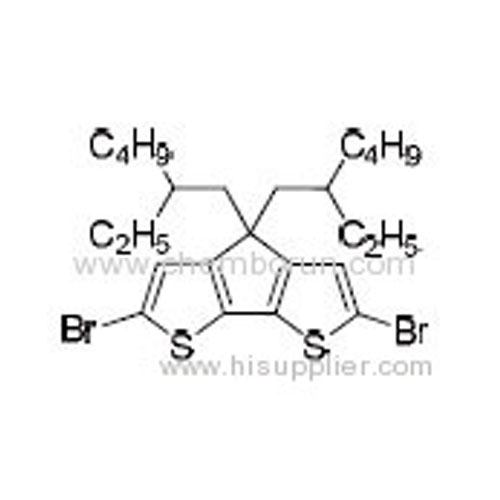
2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene -
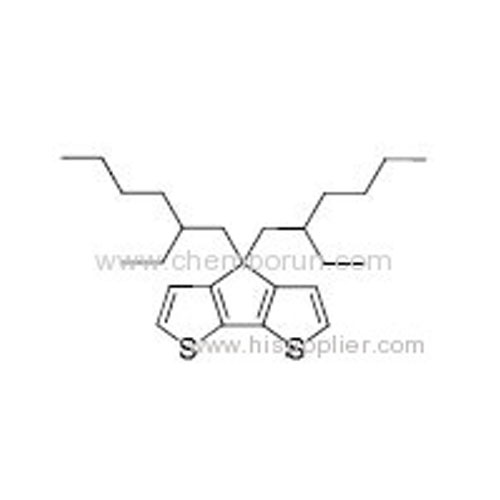
4,4-Bis(2-ethylhexyl)-4H-cyclopenta[1,2-b:5,4-b'] dithiophene -
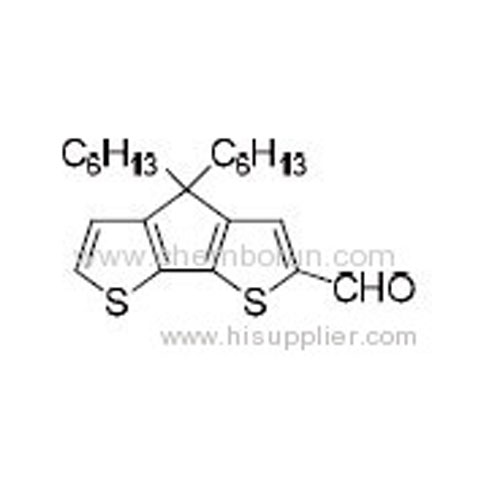
4,4-Dihexyl-4H-cyclopenta[1,2-b:5,4-b']dithiophene-2-carbaldehyde -
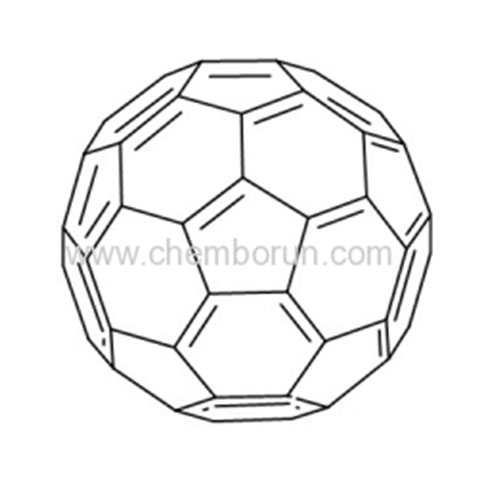
C60 sublimed grade 99.5% and 99.9% CAS 99685-96-8 -
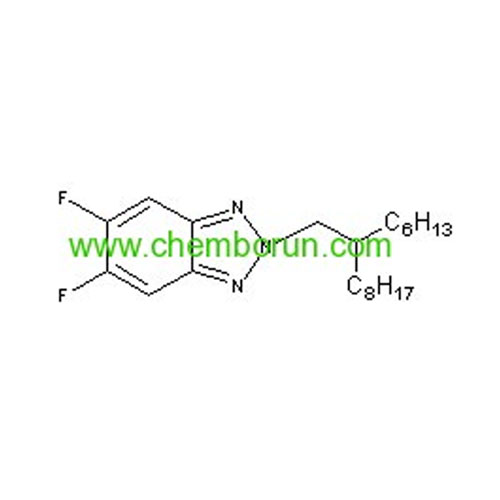
5,6-Difluoro-2-(2-hexyl-decyl)-2H-benzotriazole -
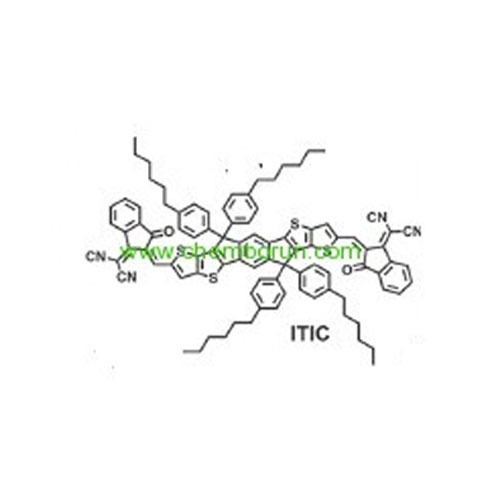
ITIC-OPV material -
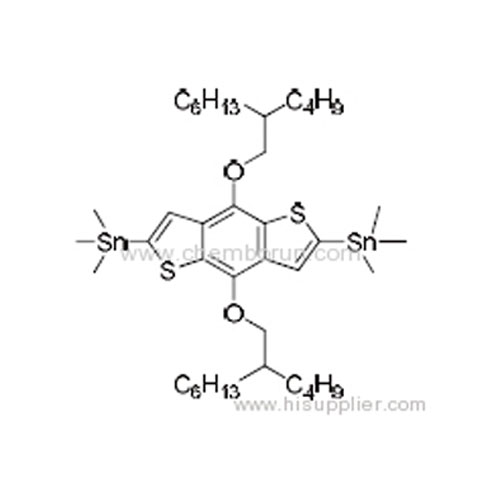
(4,8-Bis((2-butyloctyl)oxy)benzo[1,2-b:4,5-b']dithiophene-2,6-diyl)bis (trimethylstannane) -
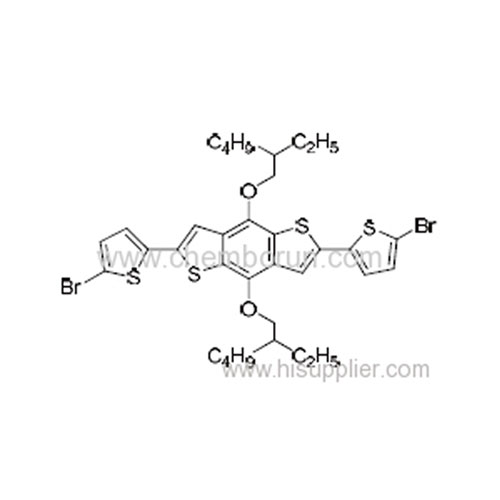
2,6-Bis(5-bromothiophen-2-yl)-4,8-bis((2-ethylhexyl)oxy)benzo[1,2-b:4,5-b'] dithiophene -
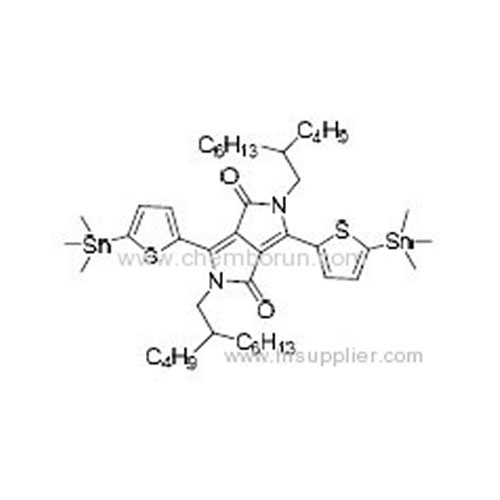
2,5-Bis(2-butyloctyl)-3,6-bis(5-(trimethylstannyl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
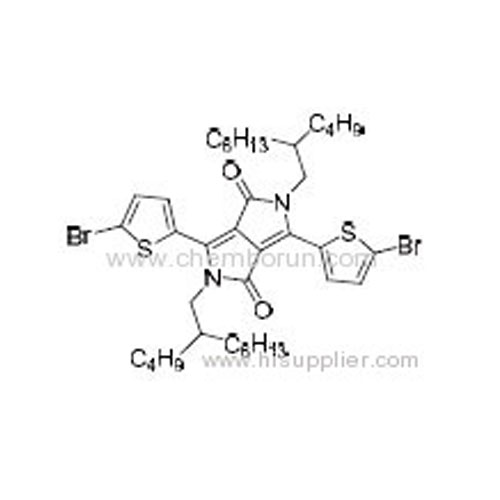
3,6-Bis(5-bromothiophen-2-yl)-2,5-bis(2-butyloctyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
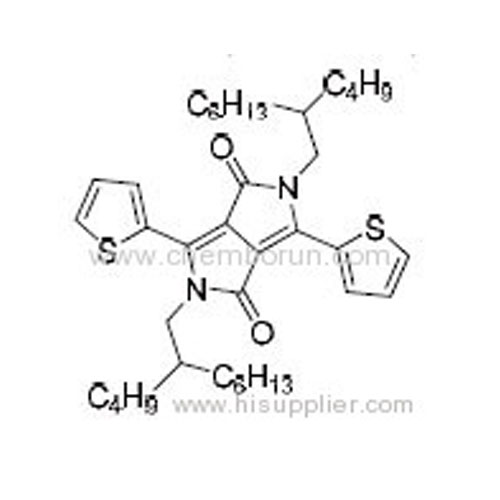
2,5-Bis(2-butyloctyl)-3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
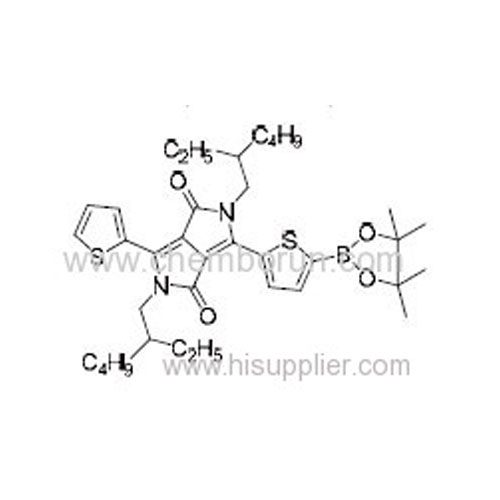
2,5-Bis(2-ethylhexyl)-3-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophen-2-yl)-6-(thiophen-2-yl)pyrrolo[3,4-c]py -
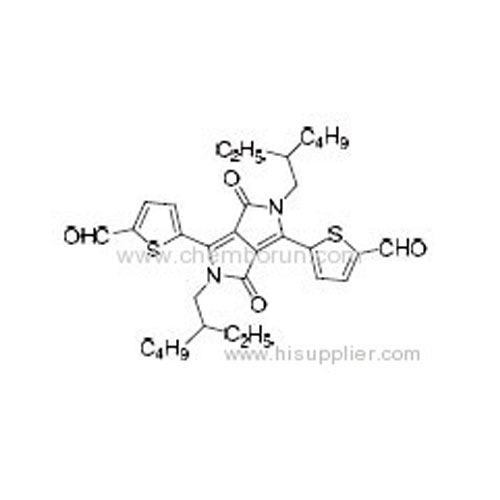
5,5'-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrole-1,4-diyl)bis(thiophene-2-carbaldehyde) -
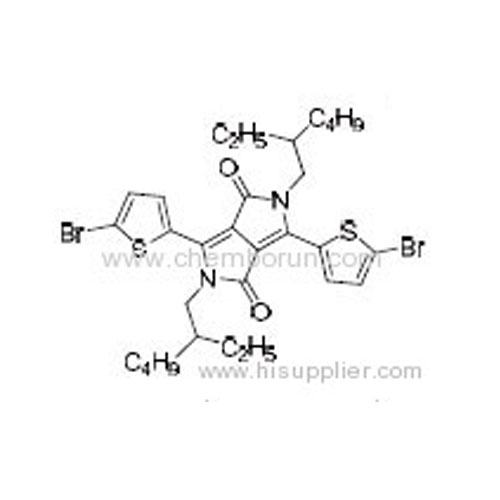
3,6-Bis(5-bromothiophen-2-yl)-2,5-bis(2-ethylhexyl)pyrrolo[3,4-c]pyrrole-1,4-dione -
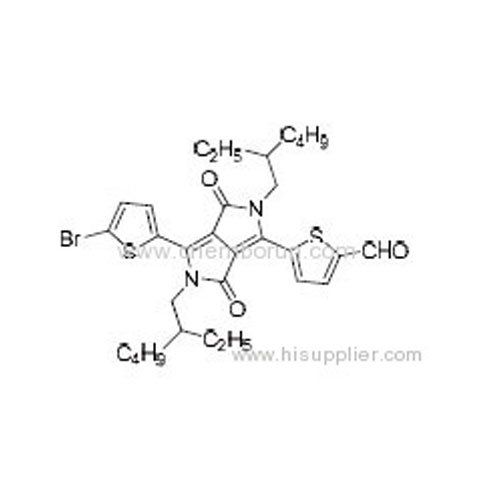
5-(4-(5-Bromothiophen-2-yl)-2,5-bis(2-ethylhexyl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophene-2-carb -
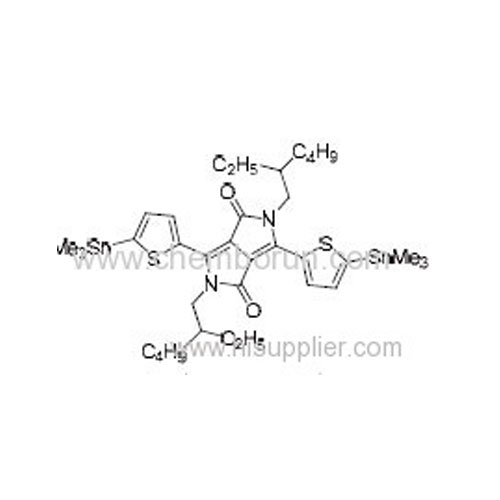
2,5-Bis(2-ethylhexyl)-3,6-bis(5-(trimethylstannyl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
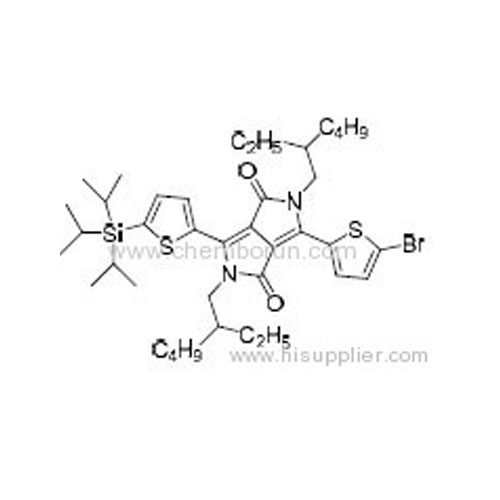
3-(5-Bromothiophen-2-yl)-2,5-bis(2-ethylhexyl)-6-(5-(triisopropylsilyl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-di -
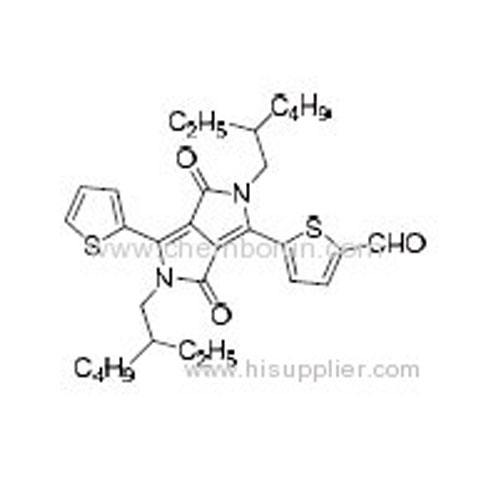
5-(2,5-Bis(2-ethylhexyl)-3,6-dioxo-4-(thiophen-2-yl)-2,3,5,6-tetrahydropyrrolo[3,4-c]pyrrol-1-yl)thiophene-2-carbaldehyd -
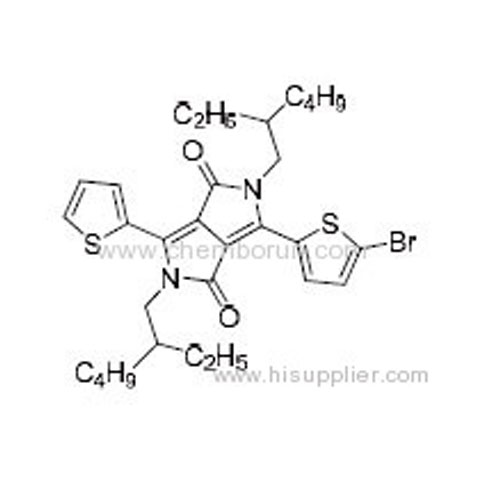
3-(5-Bromothiophen-2-yl)-2,5-bis(2-ethylhexyl)-6-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione -
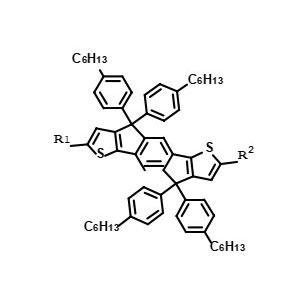
OPV Donors & Ligant -
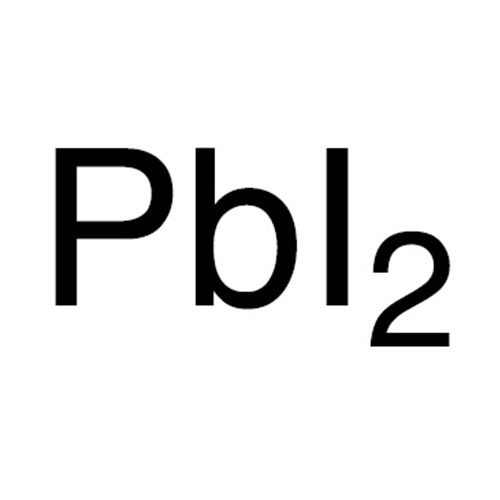
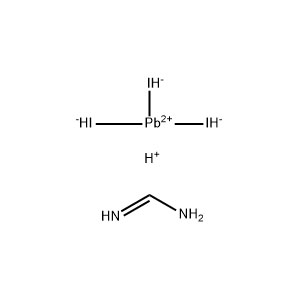
Lead iodide PbI2 -

Spiro-OMeTAD (spiro-ometad) sublimed(99.8% and 99.9% purity) -
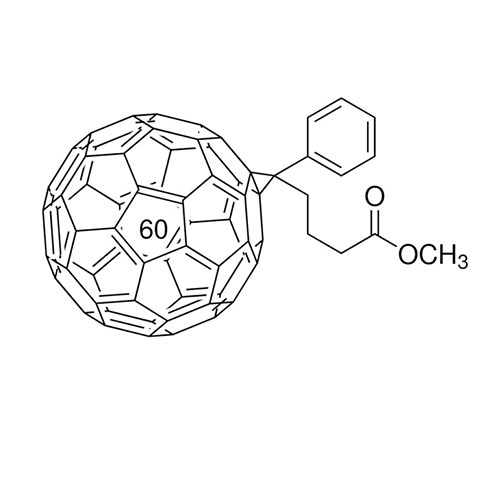
[6,6]-Phenyl C61 butyric acid methyl ester >99.5% Spiro-OMeTAD -
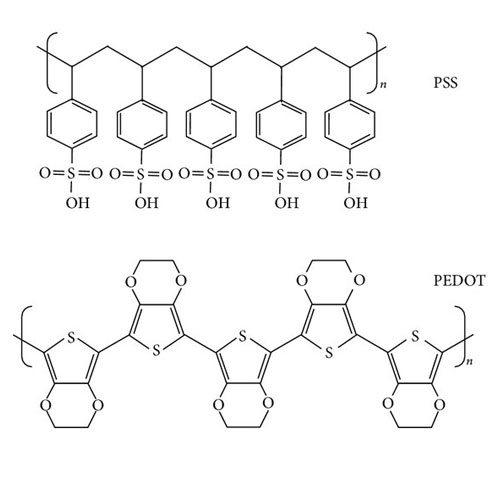
PEDOT:PSS (Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate) -
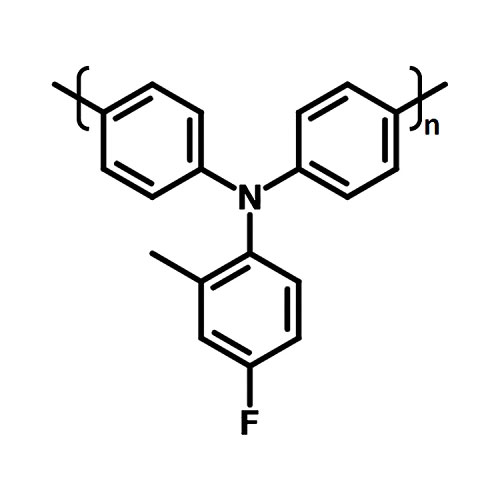
PTAA -

NiOx Nickel Oxide (NiO) Nanopowder / Nanoparticles -
FAPbI3 Microcrystalline
- HTM Material
- Perovskite Materials
- Perovskites
- Microcrystalline
- Iodides
- Bromides
- Chlorides
- Thiocyanates
- Cyanates
- Spiro
- Tetrafluoroborates
- Hexafluorophosphate
- TFSI
- Acetates
- Trifluoroacetates
- Cobalt Complexes
- Trimethyl Phosphonoacetate
- Ligands & Additives
- Ligands & Intermediates
- Additives And Modifiers
- OPV Donors & Ligant
- Perovskite Precursor
- Organic Photovoltaic (OPV)
- Spiro-OMeTAD sublimed grade
- Interface and additive materials
- Industrial Perovskite

























































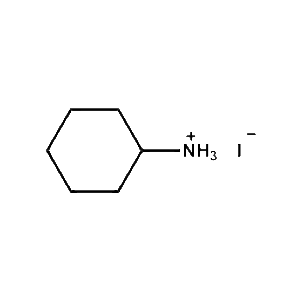

































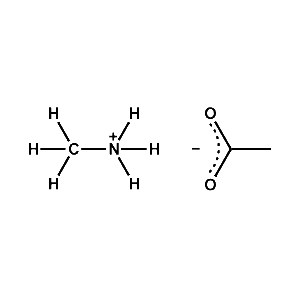


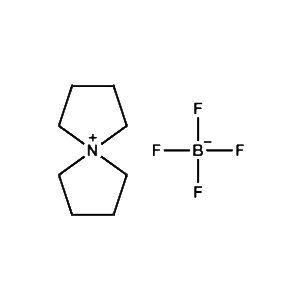










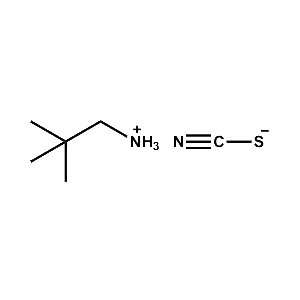













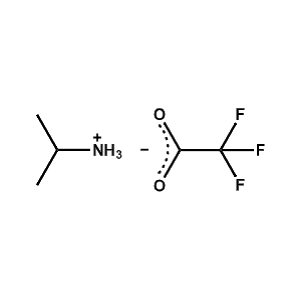

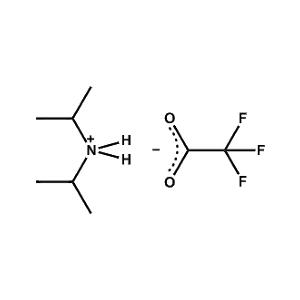


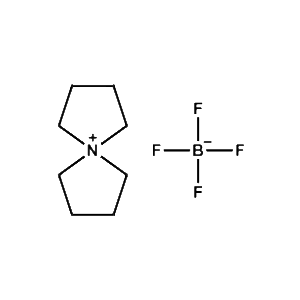










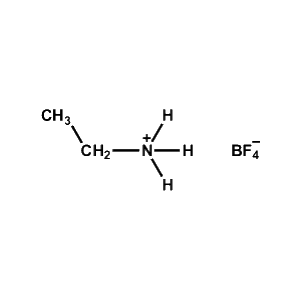
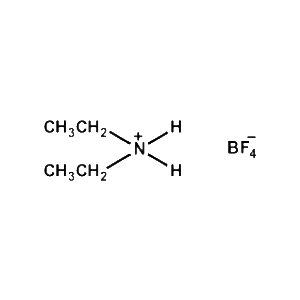



















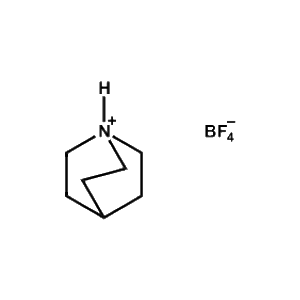





































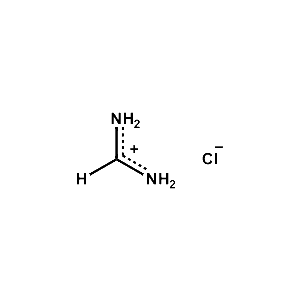
























































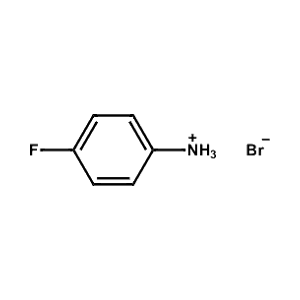























































































































































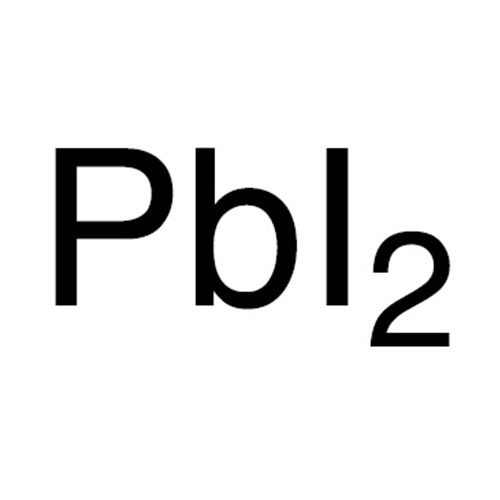






 E-mail: info@chemborun.com
E-mail: info@chemborun.com Tel: +86-574-87178138
Tel: +86-574-87178138  No. 1558, Jiangnan Road,, Ningbo, Zhejiang, China (Mainland)/31
No. 1558, Jiangnan Road,, Ningbo, Zhejiang, China (Mainland)/31